Vertex (VRTX) to Report Q1 Earnings: What's in the Cards?
Vertex Pharmaceuticals Incorporated VRTX is scheduled to release first-quarter 2024 results on May 6, after market close.
Let's see how things might have shaped up for the quarter to be reported.
Factors to Consider
Vertex currently markets four cystic fibrosis (CF) products — Trikafta/Kaftrio, Symdeko (marketed as Symkevi in Europe), Orkambi and Kalydeco.
The company’s revenues in the to-be-reported quarter are likely to have been driven by the robust uptake of its blockbuster CF medicine, Trikafta/Kaftrio (Trikafta’s brand name in Europe). Sales growth of the drug is expected to have been driven by solid demand in the international market, coupled with expanded use in children (aged between two and five years) with CF in the United States. The Zacks Consensus Estimate and our model estimate for Trikafta/Kaftrio sales are both currently pegged at $2.33 billion.
However, higher Trikafta/Kaftrio sales are likely to have caused sales erosion of Vertex’s other CF drugs.
While CF remains the main area of focus, the company saw rapid success in its non-CF pipeline candidates’ development in 2023. Investors are awaiting updates on its non-CF pipeline candidates on the first-quarter conference call.
Last year, the FDA approved Vertex and CRISPR Therapeutics’ CRSP gene therapy, Casgevy (exa-cel), for treating sickle cell disease (SCD) in patients aged 12 years and older.
Consequently, in early 2024, VRTX and CRISPR Therapeutics announced that the FDA has expanded the Casgevy label to treat transfusion-dependent beta thalassemia (TDT) in patients aged 12 years and older.
In February 2024, the European Commission approved Casgevy for the treatment of patients aged 12 years and older with severe SCD and TDT.
In the fourth quarter of 2023, Vertex said that the commercial launch of Casgevy was underway in the United States, United Kingdom, Saudi Arabia and Bahrain. We expect management to announce the first sales numbers of Casgevy on the first-quarter conference call.
Vertex is also developing treatments for acute and neuropathic pain, APOL1-mediated kidney disease (AMKD), type I diabetes and alpha-1 antitrypsin deficiency. It has earlier-stage programs in diseases, such as muscular dystrophy.
Investors are paying a lot of attention to pain asset suzetrigine (formerly known as VX-548), which, they believe, has blockbuster potential.
Around mid-April, the FDA allowed Vertex to begin a rolling new drug application (NDA) submission for suzetrigine across a broad label in moderate-to-severe acute pain. The company has already initiated the rolling submission process and is on track to complete the NDA submission in the second quarter of 2024.
Based on the successful phase II study of suzetrigine for painful diabetic peripheral neuropathy (DPN), the company is currently gearing up to initiate a pivotal phase III program of suzetrigine for the same indication in the second half of 2024. DPN is a form of peripheral neuropathic pain caused by damage to nerves. Updates from the program are also expected in the upcoming earnings release.
Earlier this month, VRTX initiated the late-stage portion of phase II/III AMPLITUDE study evaluating inaxaplin (VX-147) in AMKD, a genetic disorder affecting kidney function. The AMPLITUDE study is designed to assess the impact of inaxaplin on kidney function and proteinuria for people living with AMKD.
Earnings Surprise History
The company beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 2.74%. In the last reported quarter, the company came up with an earnings surprise of 2.44%.
Shares of Vertex have lost 2.6% in the year-to-date period compared with the industry’s decline of 10.9%.
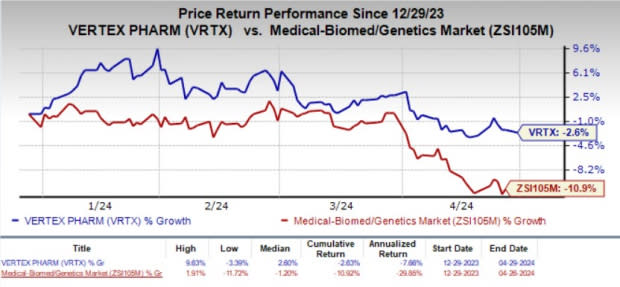
Image Source: Zacks Investment Research
Key Development
On Apr 10, 2024, Vertex announced a $4.9 billion definitive agreement to acquire Alpine Immune Sciences ALPN.
The acquisition will add Alpine’s lead asset, povetacicept (formerly ALPN-303), to Vertex’s pipeline. Povetacicept is designed to target two proteins, namely BAFF and APRIL, which are jointly responsible for the cause of multiple serious autoimmune diseases. This drug is set to enter into late-stage development for the treatment of IgA nephropathy (IgAN) in the second half of 2024. Apart from IgAN, VRTX also intends to develop Alpine’s povetacicept for multiple serious diseases, including other autoimmune kidney diseases and autoimmune cytopenias.
The transaction, expected to be completed in the second quarter of 2024, is subject to customary closing conditions and clearance from regulatory authorities. The board of directors of Alpine and Vertex has already approved this transaction.
Earnings Whispers
Our proven model does not conclusively predict an earnings beat for Vertex this time around. The combination of a positive Earnings ESP and a Zacks Rank #1 (Strong Buy), 2 (Buy), or 3 (Hold) increases the odds of an earnings beat. Unfortunately, that is not the case here, as you will see below. You can uncover the best stocks to buy or sell before they're reported with our Earnings ESP Filter.
Earnings ESP: Vertex has an Earnings ESP of -1.17%. The Most Accurate Estimate is pegged at $4.05, while the Zacks Consensus Estimate is pinned at $4.10.
Zacks Rank: VRTX currently carries a Zacks Rank #3.
Vertex Pharmaceuticals Incorporated Price and EPS Surprise
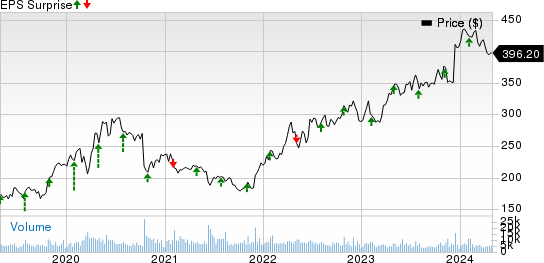
Vertex Pharmaceuticals Incorporated price-eps-surprise | Vertex Pharmaceuticals Incorporated Quote
Stock to Consider
Here is a stock worth considering from the same industry, as our model shows that it has the right combination of elements to beat on earnings this reporting cycle.
Alnylam Pharmaceuticals ALNY has an Earnings ESP of +5.87% and a Zacks Rank #3 at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Shares of Alnylam have plunged 23% year to date. ALNY beat earnings estimates in three of the last four quarters while missing the mark on one occasion, delivering an average surprise of 45.05%. The company is slated to report first-quarter 2024 results on May 2, before the opening bell.
Stay on top of upcoming earnings announcements with the Zacks Earnings Calendar.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
Alpine Immune Sciences, Inc. (ALPN) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 