Fennec Pharmaceuticals Inc. (FRX.TO)
Toronto - Toronto Real Time Price. Currency in CAD
Add to watchlist
As of 01:16PM EDT. Market open.
| Previous Close | 12.80 |
| Open | 12.75 |
| Bid | 12.57 x 0 |
| Ask | 12.67 x 0 |
| Day's Range | 12.75 - 12.81 |
| 52 Week Range | 9.27 - 15.43 |
| Volume | |
| Avg. Volume | 280 |
| Market Cap | 346.879M |
| Beta (5Y Monthly) | 0.40 |
| PE Ratio (TTM) | N/A |
| EPS (TTM) | -0.82 |
| Earnings Date | May 09, 2024 - May 13, 2024 |
| Forward Dividend & Yield | N/A (N/A) |
| Ex-Dividend Date | N/A |
| 1y Target Est | N/A |
 Simply Wall St.
Simply Wall St.Those who invested in Fennec Pharmaceuticals (NASDAQ:FENC) five years ago are up 136%
When you buy a stock there is always a possibility that it could drop 100%. But when you pick a company that is really...
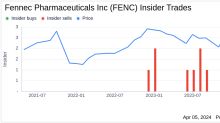 GuruFocus.com
GuruFocus.comInsider Sell: CEO Rosty Raykov Sells 88,583 Shares of Fennec Pharmaceuticals Inc (FENC)
Fennec Pharmaceuticals Inc (NASDAQ:FENC), a biopharmaceutical company focused on the development of PEDMARK for the prevention of platinum-induced ototoxicity in pediatric cancer patients, has reported a significant insider sell transaction.
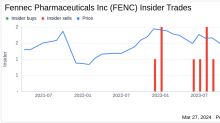 GuruFocus.com
GuruFocus.comChief Financial Officer Robert Andrade Sells 13,975 Shares of Fennec Pharmaceuticals Inc (FENC)
Robert Andrade, the Chief Financial Officer of Fennec Pharmaceuticals Inc (NASDAQ:FENC), has sold 13,975 shares of the company on March 26, 2024, according to a recent SEC Filing.