Applied Therapeutics, Inc. (APLT)
After hours:
| Previous Close | 4.6200 |
| Open | 4.7000 |
| Bid | 4.3800 x 400 |
| Ask | 4.4100 x 200 |
| Day's Range | 4.3450 - 4.7050 |
| 52 Week Range | 1.1800 - 9.3900 |
| Volume | |
| Avg. Volume | 1,752,430 |
| Market Cap | 527.797M |
| Beta (5Y Monthly) | N/A |
| PE Ratio (TTM) | N/A |
| EPS (TTM) | -1.4200 |
| Earnings Date | Aug 08, 2024 - Aug 12, 2024 |
| Forward Dividend & Yield | N/A (N/A) |
| Ex-Dividend Date | N/A |
| 1y Target Est | 13.05 |
 GuruFocus.com
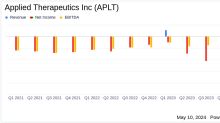
GuruFocus.comApplied Therapeutics Inc (APLT) Reports Q1 2024 Earnings: A Deep Dive into Financials and ...
Exploring the Financial Landscape and Future Prospects Amidst Regulatory Advances
 GlobeNewswire
GlobeNewswireApplied Therapeutics Reports First Quarter 2024 Financial Results
Govorestat NDA for Classic Galactosemia under Priority Review, PDUFA target action date of November 28, 2024 Govorestat MAA under review by EMA; Day 120 3-month clock-stop extension granted; decision expected in early Q1 2025Company discussing potential NDA submission under Accelerated Approval for govorestat for treatment of SORD Deficiency with Neurology I Division of FDA NEW YORK, May 09, 2024 (GLOBE NEWSWIRE) -- Applied Therapeutics, Inc. (Nasdaq: APLT) (the “Company”), a clinical-stage biop
 GlobeNewswire
GlobeNewswireApplied Therapeutics to Present at the 2024 RBC Capital Markets Global Healthcare Conference
NEW YORK, May 07, 2024 (GLOBE NEWSWIRE) -- Applied Therapeutics, Inc. (Nasdaq: APLT), a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need, today announced that it will participate in a fireside chat at the 2024 RBC Capital Markets Global Healthcare Conference on Tuesday, May 14, 2024 at 4:35 p.m. ET in New York, New York. A live webcast for this event will be accessible on the Even