Regeneron's (REGN) Lymphoma Candidate Rejected by the FDA
Regeneron Pharmaceuticals, Inc. REGN suffered a setback as the FDA issued Complete Response Letters (CRLs) for its biologics license application (BLA) for odronextamab.
Odronextamab is an investigational bispecific monoclonal antibody designed to bind to a component of the T-cell receptor complex while binding and bridging T-cells to a protein expressed on B-cells.
The BLA is seeking the approval of the candidate in relapsed/refractory (R/R) follicular lymphoma (FL) and in R/R diffuse large B-cell lymphoma (DLBCL), each after two or more lines of systemic therapy.
Regeneron stated that the sole issue with approvability is related to the enrollment status of the confirmatory trials.
The CRLs – one for R/R FL and one for R/R DLBCL – did not identify any approvability issues with the clinical efficacy or safety, trial design, labeling or manufacturing of the candidate.
We remind investors that REGN has been actively enrolling patients in multiple phase III trials for odronextamab as part of the OLYMPIA program.
The program is expected to change the treatment paradigm of several B-cell non-Hodgkin lymphoma subtypes, including in earlier lines of therapy.
The FDA agreed to the OLYMPIA program and required that the trials include both dose-finding and confirmatory portions.
While enrollment has already begun in the dose-finding portion of the studies, the CRLs indicate that the confirmatory portions of these trials should be underway currently. The regulatory body also needs to agree to timelines prior to resubmission.
Regeneron targets sharing updates on enrollment status and regulatory timelines later in 2024.
Meanwhile, the candidate is also under review in the European Union (“EU”) for the treatment of R/R DLBCL and R/R FL. Odronextamab enjoys Orphan Drug Designation in DLBCL and FL in the EU.
The CRL delays the potential approval of the candidate and REGN’s plans to expand its oncology portfolio, which currently comprises antibody Libtayo.
Regeneron’s shares have gained 17.1% in the past year against the industry’s decline of 8.4%.
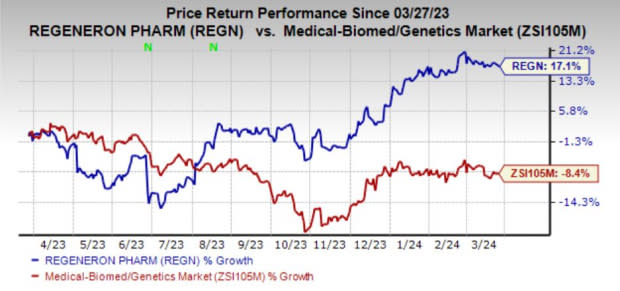
Image Source: Zacks Investment Research
Libtayo is approved for five different cancers (basal cell carcinoma, lung cancer, cervical cancers and others).
Regeneron is evaluating Libtayo as monotherapy and in combination with either conventional or novel therapeutic approaches in various solid tumors and blood cancers.
The FDA has also accepted for Priority Review the BLA for Regeneron’s linvoseltamab to treat adult patients with R/R multiple myeloma (MM) that has progressed after at least three prior therapies. The target action date is Aug 22, 2024.
Last month, Regeneron’s fourth-quarter sales and earnings beat estimates, but the lead drug Eylea’s sales declined year over year in the fourth quarter.
Regeneron Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
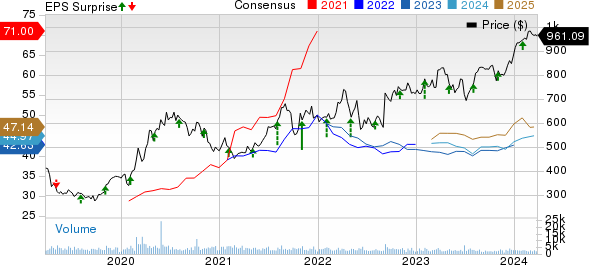
Regeneron Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Regeneron Pharmaceuticals, Inc. Quote
Eylea sales were under pressure in 2023 due to competition from Roche’s Vabysmo.
Nevertheless, REGN should benefit from Dupixent’s continued label expansions and solid demand. The approval of Eylea HD is also a great boost, generating incremental revenues for the company.
REGN currently has a Zacks Rank #3 (Hold).
Stocks to Consider
Some better-ranked stocks in the healthcare sector are ADMA Biologics, Inc. ADMA, Galapagos GLPG and ANI Pharmaceuticals, Inc. ANIP, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share have improved from 22 to 30 cents. In the past year, shares of ADMA have rallied 107.4%.
ADMA Biologics’ earnings beat estimates in three of the trailing four quarters and met once, delivering an average surprise of 85.00%.
In the past 60 days, estimates for GLPG’s loss have narrowed from $1.68 per share to 39 cents. Galapagos beat on earnings in three of the trailing four quarters and missed once, delivering an average surprise of 92.4%.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have improved from $4.06 to $4.43. In the past year, shares of ANIP have surged 81.3%.
ANI Pharmaceuticals’ earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 109.06%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Galapagos NV (GLPG) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 