PTC Therapeutics (PTCT) Up as EC Returns CHMP Opinion on DMD Drug
PTC Therapeutics, Inc. PTCT announced that the European Commission (EC) has decided against adopting the Committee for Medicinal Products for Human Use’s (CHMP) negative opinion of January 2024 on the annual renewal of the conditional marketing authorization of Translarna (ataluren).
Translarna is a protein restoration therapy designed to enable the formation of a functioning protein in patients with genetic disorders caused by a nonsense mutation.
Following the decision, the EC has returned the opinion to the CHMP for re-evaluation. Thus, in the meantime, Translarna remains available in the market for patients with nonsense mutation Duchenne muscular dystrophy (nmDMD) in the EU, per its current marketing authorization.
The EC has also instructed the CHMP to further consider the totality of evidence, including data from patient registries and real-world evidence, in a revised opinion.
nmDMD is a genetic, rare and fatal muscle-wasting condition that typically affects males. It causes progressive muscle weakness from early childhood and leads to premature death in the mid-20s due to heart and respiratory failure.
Management claims this unprecedented decision by the EC is a “big win” for patients suffering from nmDMD as no other disease-modifying therapies are currently available for this indication.
PTC Therapeutics’ stock soared 21.2% during the last trading session on May 20, as the investors cheered the regulatory development. Year to date, shares of PTCT have rallied 45.6% against the industry’s 7.2% decline.
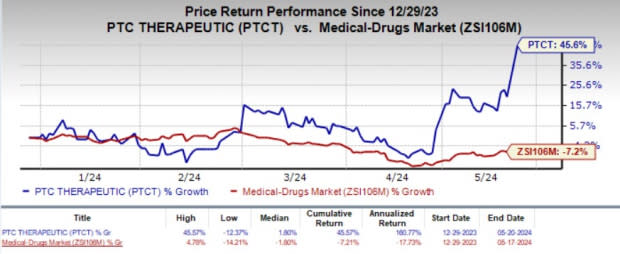
Image Source: Zacks Investment Research
The EC has also informed the company that it has decided to consider the Scientific Advisory Group (SAG) meeting for Translarna held on Sep 5, 2023, and all the following procedural steps as invalid. Additionally, the input from the September 2023 SAG meeting as well as the meeting held in January 2024, will not be considered by the CHMP in any future evaluation of Translarna.
At present, PTC Therapeutics is gearing up to collaborate with the CHMP on the next steps regarding the revaluation of the conditional marketing authorization of Translarna for nmDMD once they are defined.
However, given the uncertainty regarding the continued authorization of Translarna in Europe, the company is unable to accurately forecast its impact on 2024 revenues. Hence, PTCT has currently abstained from providing a 2024 total revenue guidance.
The company expects to update the guidance at a future date.
In the United States, PTC Therapeutics plans to re-submit a new drug application (NDA) for Translarna for the treatment of nmDMD based on feedback from the FDA received earlier this year.The NDA resubmission is targeted by the middle of 2024.
We remind the investors that the NDA pathway for potential Translarna approval in the United States has been nothing short of a rocky ride so far.
The NDA for Translarna was filed initially in 2017. However, the FDA issued a complete response letter in October 2017, stating that it was unable to approve the application in its current form.
After numerous discussions, meetings and FDA’s request for additional data, PTCT held a type C meeting with the FDA in the fourth quarter of 2023 to discuss the totality of Translarna data. Based on this discussion, the FDA suggested PTCT to request a pre-submission type C meeting to discuss the specific contents of an NDA resubmission based on the results from Study 041 and its long-term, international drug registry study (STRIDE) for nmDMD patients receiving Translarna.
PTC Therapeutics, Inc. Price and Consensus
PTC Therapeutics, Inc. price-consensus-chart | PTC Therapeutics, Inc. Quote
Zacks Rank and Stocks to Consider
PTC Therapeutics currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are Ligand Pharmaceuticals LGND, ANI Pharmaceuticals ANIP and Annovis Bio ANVS. Each stock presently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Ligand’s 2024 earnings per share (EPS) has remained constant at $4.56. During the same time frame, the consensus estimate for 2025 EPS has remained constant at $5.27. Year to date, shares of LGND have gained 21.3%.
Ligand beat estimates in each of the trailing four quarters, delivering an average surprise of 56.02%.
In the past 30 days, estimates for ANI Pharmaceuticals’ 2024 EPS have risen from $4.43 to $4.44. During the same period, the consensus estimate for 2025 EPS has remained constant at $5.04. Year to date, shares of ANIP have rallied 12%.
ANI Pharmaceuticals beat estimates in each of the last four quarters, delivering an average surprise of 53.90%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $3.35 to $2.46. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.82 to $1.95. Year to date, shares of ANVS have plunged 53.2%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
PTC Therapeutics, Inc. (PTCT) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 