Jazz Pharma's (JAZZ) Gets FDA Nod for Rylaze's New Dosing Option
Jazz Pharmaceuticals JAZZ recently announced that the FDA approved a supplemental biologics license application (sBLA) seeking approval for the addition of a Monday/Wednesday/Friday (“MWF”) intramuscular (IM) dosing schedule for its novel asparaginase, Rylaze
The label expansion of Rylaze to include the MWF dosing option was approved by the FDA under the Real-Time Oncology Review (RTOR) program.
The company had submitted the sBLA seeking approval for an MWF IM dosing schedule for Rylaze in April 2022.
Rylaze is already approved by the FDA under its RTOR program for use in the United States as a component of a multi-agent chemotherapeutic regimen for treating acute lymphoblastic leukemia (“ALL”) or lymphoblastic lymphoma (“LBL”) in adults and children one month or older with hypersensitivity to E. coli-derived asparaginase.
The approval of the MWF dosing option was based on the data from the third cohort of phase II/III study (JZP458-201 or AALL1931), which evaluated the IM route of administration of Rylaze, including the Monday-Wednesday-Friday dosing schedule.
Results from the study exhibited a positive benefit-to-risk profile of the drug when a dosing regimen of 25 mg/m2 administered intramuscularly on Monday and Wednesday and 50 mg/m2 administered on Friday was followed.
Rylaze also maintained a consistent safety profile throughout the duration of the treatment, in line with the safety parameters of ALL/LBL patients.
Shares of Jazz Pharmaceuticals have returned 15.5% in the year-to-date period against the industry’s decline of 31%.
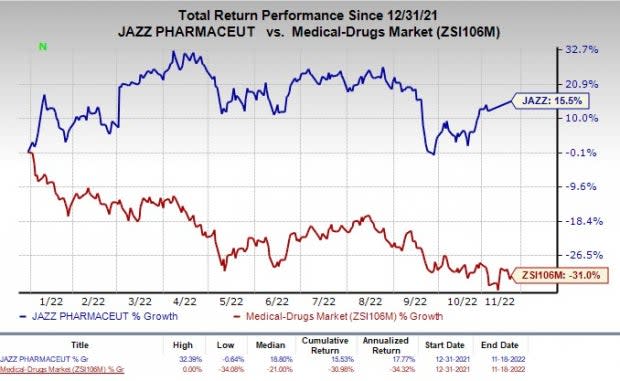
Image Source: Zacks Investment Research
Jazz completed the submission of an sBLA with the European Medicines Agency (EMA) seeking approval for the intravenous administration of Rylaze. The company also submitted a and marketing authorization application (MAA) with the European regulatory body.
Jazz has a strong oncology portfolio with several hematology/oncology drugs in its portfolio, aiding growth and diversification. It includes two older drugs, namely, Defitelio for treating patients with the hepatic veno-occlusive disease (VOD) with renal or pulmonary dysfunction, and Vyxeos for treating adultys with two types of acute myeloid leukemia (AML). Its oncology portfolio includes two newer drugs, one is Rylaze and the other is Zepzelca (lurbinectedin), approved in 2020 for treating patients with metastatic small cell lung cancer (SCLC).
Jazz Pharmaceuticals, in August 2022, entered into a licensing deal with Zymeworks ZYME for the latter’s HER-2 targeted bispecific antibody, zanidatamab. Jazz continues to expand its oncology profile with the addition of the novel late-stage asset, zanidatamab, with compelling anti-tumor activity.
Per the agreement, Jazz will acquire the rights to develop and commercialize zanidatamab across all its indications in all territories except in Asia Pacific, where the drug is already licensed toBeiGene. In return, Jazz will make an upfront payment of $50 million to Zymeworks, once the transaction is approved.
Zanidatamab is currently being developed by Zymeworks in several phase III studies for treating biliary tract cancer and gastroesophageal adenocarcinomas. Top-line data from the phase III biliary tract cancer study is expected by the end of 2022.
Jazz Pharmaceuticals PLC Price
Jazz Pharmaceuticals PLC price | Jazz Pharmaceuticals PLC Quote
Zacks Rank and Key Picks
InMed Pharmaceuticals currently has a Zacks Rank #3 (Hold)
Some better-ranked stocks in the same sector include Assertio ASRT and Esperion Therapeutics ESPR, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Assertio earnings per share estimates for 2022 have improved by a cent to 52 cents in the past 30 days. The same for 2023 has improved from 33 cents to 44 cents in the same time frame.
Earnings of Assertio beat estimates in three of the trailing four quarters, while beating the same in the reaming occasion. The average earnings surprise for ASRT is 54.96%.
Esperion’s loss per share estimates for 2022 have narrowed from $3.82 to $3.66 in the past 30 days. The loss per share for 2023 has narrowed from $1.89 to $1.67 in the same time frame.
Earnings of Esperion beat estimates in three of the trailing four quarters, while missing the same on the remaining occasion. The average earnings surprise for ESPR is 7.30%
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Jazz Pharmaceuticals PLC (JAZZ) : Free Stock Analysis Report
Esperion Therapeutics, Inc. (ESPR) : Free Stock Analysis Report
Zymeworks Inc. (ZYME) : Free Stock Analysis Report
Assertio Holdings, Inc. (ASRT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 