Horizon (HZNP) Up 36.7% YTD: Pipeline Development in Focus
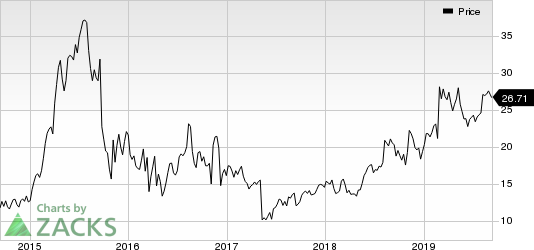
Shares of Horizon Therapeutics plc HZNP have surged 36.7% year to date against the industry’s decline of 1.9%.

The company has been focusing on expanding its orphan drug business. This is evident from its long-range plan, wherein the orphan drug business is expected to constitute approximately 60% of net sales in 2020. The company has been quite active on the acquisition front over the last few quarters.
Earlier in the month, the FDA accepted the Biologics License Application (BLA) for the company’s investigational medicine, teprotumumab, for the treatment of active thyroid eye disease (TED), and granted it Priority Review designation. If approved, teprotumumab would be the first FDA-approved medicine for the treatment of active TED. Teprotumumab is a fully human monoclonal antibody (mAb) and a targeted inhibitor of the IGF-1R. The candidate was added to the company’s portfolio after the acquisition of River Vision in 2017. The company submitted a BLA to the FDA for teprotumumab in July 2019. If approved, this will boost sales for the company.
Horizon is working on the label expansion of its marketed drugs — Procysbi, Actimmune and Ravicti.
This July, the company announced the FDA's acceptance of its new drug application (NDA) for Procysbi (Cysteamine Bitartrate) delayed-release oral granules in packets. If approved, this new dosage form will be another administration option for patients, in addition to the currently available Procysbi delayed-release capsules. These capsules are FDA-approved for children aged one year or older and adults living with nephropathic cystinosis. The agency is expected to make a decision in 2020.
Actimmune is currently approved for the treatment of chronic granulomatous disease (CGD) and severe malignant osteopetrosis (SMO). Horizon is working to increase awareness about the drug among patients and physicians. An investigator-initiated study at the Moffitt Cancer Center is underway and enrolling patients. The study is evaluating Actimmune in combination with Roche Holding AG’s RHHBY Herceptin, Perjeta and Taxol and aims to determine the optimal dosing and treatment combination in certain advanced breast cancer patients. The company has collaborated with the Fox Chase Cancer Center to evaluate the drug in combination with Bristol-Myers Squibb Company’s BMY Opdivo in a phase I dosing study for the treatment of kidney and bladder cancer. The National Cancer Institute is evaluating Actimmune in combination with Merck & Co’s MRK Keytruda in a phase II study to treat cutaneous t-cell lymphoma patients. Also, a dose ranging study is evaluating the drug in combination with Bristol-Myers’ Taxol and Roche’s Herceptin and Perjeta in a certain type of advanced breast cancer.
During the first half of 2019, the company generated double-digit net sales growth in its orphan and rheumatology segment, driven by continued momentum from Krystexxa, its medicine for uncontrolled gout and the main growth driver. Based on this, the company raised its guidance for full-year 2019 net sales to $1.28-$1.30 billion from the previous guidance of $1.26-$1.28 billion.
Horizon currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Horizon Pharma Public Limited Company Price
Horizon Pharma Public Limited Company price | Horizon Pharma Public Limited Company Quote
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +98%, +119% and +164% in as little as 1 month. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Horizon Pharma Public Limited Company (HZNP) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 