Glaxo Begins Phase III Study on Injection to Prevent HIV
GlaxoSmithKline plc GSK has announced the start of a large late-stage African study on an experimental long-acting injection for the prevention of HIV. ViiV Healthcare, an HIV company majorly owned by Glaxo and Pfizer PFE, said that the phase III study, HPTN 084, will evaluate long-acting cabotegravir monotherapy for the prevention of HIV infection in sexually active women.
ViiV Healthcare is conducting the study under a public private funding collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Bill & Melinda Gates Foundation. While NIAID, part of the National Institutes of Health (NIH), is sponsoring the study, ViiV Healthcare and Gilead GILD are providing the medicines. The study will enrol more than 3,000 women between the age of 18 and 45 from sub-Saharan African countries.
In order to prevent HIV infection, uninfected people, at present, use oral anti-retroviral medication, which needs to be taken every day. Compared to these, cabotegravir injections will be administered every two months, if approved
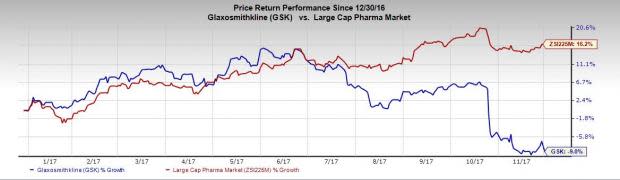
Glaxo’s shares have declined 9% this year so far, underperforming the 16.2% increase witnessed by the industry.
ViiV Healthcare is already conducting a HPTN 083 study on cabotegravir in HIV-uninfected men and transgender women who have sex with men, also under co-funding with NIAID. Meanwhile, cabotegravir is also being evaluated in three late stage studies as a two drug regimen with Johnson & Johnson’s JNJ Edurant/rilpivirine, with the third study initiation announced earlier this week.
Last week Glaxo and partner J&J announced FDA approval of the first dual treatment for HIV, Juluca (Tivicay/dolutegravir + Edurant/rilpivirine). Juluca has been developed under a partnership between ViiV Healthcare and Janssen.
HIV has been a key therapeutic area for Glaxo. Its two new HIV drugs, Tivicay and Triumeq have been recording consistently strong sales while gaining market share. Glaxo’s HIV sales rose 16% at constant exchange rate in the first nine months of 2017. Meanwhile, a significant portion of its R&D expenditures goes toward developing HIV medicines to treat/prevent HIV.
Glaxohas a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Medical Stocks to Buy Now
Zacks names 5 companies poised to ride a medical breakthrough that is targeting cures for leukemia, AIDS, muscular dystrophy, hemophilia, and other conditions.
New products in this field are already generating substantial revenue and even more wondrous treatments are in the pipeline. Early investors could realize exceptional profits.
Click here to see the 5 stocks >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer, Inc. (PFE) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
GlaxoSmithKline PLC (GSK) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 