Gilead (GILD) Resubmits NDA for HIV Inhibitor to the FDA
Gilead Sciences, Inc. GILD has announced that it resubmitted its new drug application (NDA) to the FDA for experimental candidate lenacapavir.
The company is seeking approval of lenacapavir, an investigational, long-acting HIV-1 capsid inhibitor, for treating heavily treatment-experienced (HTE) people with multi-drug-resistant (MDR) HIV-1 infection.
The resubmission comes in response to the complete response letter (CRL) issued by the agency in February 2022.
The CRL cited Chemistry Manufacturing and Controls (CMC) issues relating to the compatibility of lenacapavir in borosilicate vials.
The NDA resubmission contains comprehensive CMC data to support the compatibility of lenacapavir with an alternative vial type made from aluminosilicate glass. In addition, the application is supported by extensive pre-clinical and earlier clinical research data as well as data from the phase II/III CAPELLA study.
The study evaluated the antiviral activity of lenacapavir administered every six months as a subcutaneous injection, in combination with other antiretroviral(s), in HTE people with multi-drug resistant HIV-1 infection.
A new Prescription Drug User Fee Act (PDUFA) date will be set once the regulatory body accepts the NDA.
A potential approval will make lenacapavir the first and the only HIV-1 treatment option administered twice yearly.
The Committee for Medicinal Products for Human Use of the European Medicines Agency recently adopted a positive opinion for pipeline candidate lenacapavir. Gilead is seeking the European Commission’s approval for the candidate for the treatment of HIV-1 infection, in combination with other antiretroviral(s), in adults with multi-drug resistant HIV-1 infection for whom it is otherwise not possible to construct a suppressive antiviral regimen.
Last month, the FDA lifted the clinical hold placed on the company’s Investigational New Drug Application (IND) to evaluate injectable lenacapavir for HIV treatment and HIV pre-exposure prophylaxis (PrEP).
The stock has lost 13.4% so far in the year compared with the industry's decline of 21.7%.
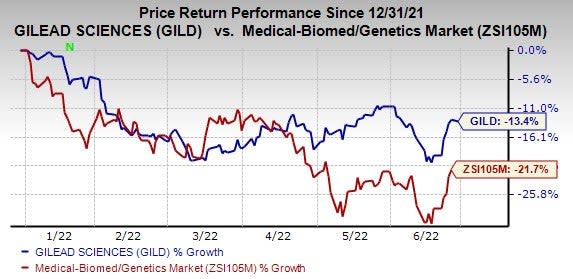
Image Source: Zacks Investment Research
Gilead has a strong and diverse portfolio of HIV drugs and therapies led by Biktarvy and Descovy. In the first quarter, sales of HIV products accounted for approximately 57% of total product sales. Biktarvy sales increased 18% year over year primarily due to higher demand.
However, competition is stiffening in this space from the likes of GlaxoSmithKline GSK, among others. The loss of exclusivity for Truvada has also hurt the company’s business.
Hence, the successful development of new innovative therapies for HIV will help Gilead maintain its dominant position in this space.
We note that Glaxo’s HIV franchise recorded 14% growth in the first quarter. Growth was driven by new HIV products like Dovato, Cabenuva, Rukobia, Juluca and Apretude and phasing.
Gilead currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks are Sesen Bio SESN and Geron Corporation GERN. While Sesen sports a Zacks Rank #1 (Strong Buy), Geron has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss estimates for SESN for 2022 have narrowed to 44 cents from a loss of 46 cents in the past 60 days. Sesen surpassed estimates in all of the trailing four quarters, the average surprise being 69.94%.
Loss estimates for GERN for 2022 have narrowed by 6 cents in the past 60 days. Geron surpassed estimates in three of the trailing four quarters, the average surprise being 1.07%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Geron Corporation (GERN) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
SESEN BIO, INC. (SESN) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 