Eli Lilly's (LLY) Efsitora Matches Daily Insulins in A1C Control
Eli Lilly’s LLY two phase III studies on its once-weekly insulin, efsitora alfa, met their primary endpoint of non-inferior reduction in blood sugar levels compared with daily basal insulins.
The patient population in the first phase III study, QWINT-2, comprised type II diabetes (T2D) patients who had never been treated with insulin before (insulin naïve). On the other hand, the phase III QWINT-4 study enrolled adults with T2D who have previously been treated with basal insulin and at least two injections per day of mealtime insulin.
In both studies, treatment with efsitora resulted in non-inferior A1C reduction compared with the most commonly used daily basal insulins globally. A1C is a test that measures the average blood sugar levels over the past three months.
Eli Lilly claims this to be a significant milestone for the diabetes community as the results demonstrate efsitora’s potential as a novel once-weekly drug that controls blood sugar levels while reducing the treatment burden of daily injections, which could lead to increased patient compliance.
Year to date, shares of LLY have jumped 32.3% compared with the industry’s 16.2% growth.
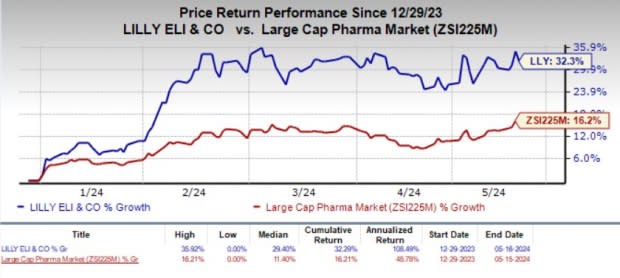
Image Source: Zacks Investment Research
In the QWINT-2 study, insulin-naïve T2D patients were randomized to receive either a dose of once-weekly efsitora or once-daily insulin degludec over a 52-week treatment period. The study was also designed to assess efficacy in patients using and not using GLP-1 receptor agonists.
In the study, efsitora reduced A1C by 1.34%, while insulin degludec reduced the same by 1.26%, resulting in an A1C of 6.87% and 6.95%, respectively.
The study also met a key secondary endpoint that asserted efsitora’s non-inferiority to insulin degludec in A1C change among patients using and not using GLP-1 receptor agonists.
The QWINT-4 study, however, evaluated the safety and efficacy of efsitora compared with insulin glargine in the previously treated T2D patient population over a 26-week treatment period. The enrolled patients were randomized into two groups to receive either efsitora once weekly or insulin glargine once daily, both in combination with insulin lispro.
Both treatment groups in the QWINT-4 study achieved an AIC reduction of 1.07%, resulting in an A1C of 7.12% and 7.11%, respectively.
The candidate was observed to be generally safe and overall well-tolerated in both the late-stage studies.
Eli Lilly expects to share detailed results from the QWINT-2 and QWINT-4 studies at an upcoming medical conference. The company further anticipates top-line data readouts from the phase III QWINT-1, QWINT-3 and QWINT-5 registrational studies, evaluating efsitora in different diabetes patient populations, later in 2024.
Eli Lilly and Company Price and Consensus
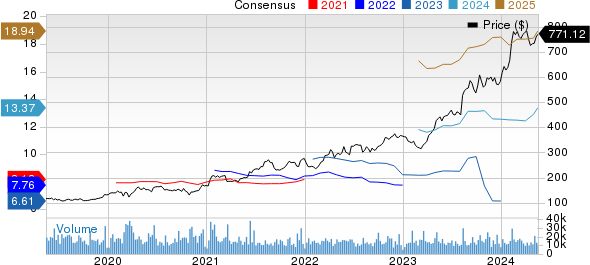
Eli Lilly and Company price-consensus-chart | Eli Lilly and Company Quote
Zacks Rank and Stocks to Consider
Eli Lilly currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are Ligand Pharmaceuticals LGND, ANI Pharmaceuticals ANIP and Annovis Bio ANVS, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Ligand’s 2024 earnings per share has remained constant at $4.56. During the same time frame, the consensus estimate for 2025 earnings per share has remained constant at $5.27. Year to date, shares of LGND have gained 19%.
Ligand beat estimates in each of the trailing four quarters, delivering an average surprise of 56.02%.
In the past 30 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have risen from $4.43 to $4.44. Meanwhile, during the same period, the consensus estimate for 2025 earnings per share has remained constant at $5.04. Year to date, shares of ANIP have climbed 15.7%.
ANI Pharmaceuticals beat estimates in each of the last four quarters, delivering an average surprise of 53.90%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $3.35 to $2.93. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.82 to $2.70. Year to date, shares of ANVS have plunged 57.1%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 