Apellis (APLS) Up on Upbeat GA Data From Long-Term Syfovre Study
Apellis Pharmaceuticals APLS announced positive data from the late-stage GALE long-term extension study evaluating its marketed drug, Syfovre (pegcetacoplan injection), to treat patients with geographic atrophy (GA) secondary to age-related macular degeneration.
Per the data readout, presented at a recent medical conference, treatment with Syfovre in the GALE study preserved visual function at 36 months in the GA patients. Per Apellis, Syfovre is the only approved GA treatment to show a benefit on visual function in a prespecified endpoint.
Additionally, it was observed that patients developed fewer new scotomatous points with 36 months of both continuous monthly and every-other-month Syfovre treatment compared with patients from the sham crossover group, which met a prespecified microperimetry endpoint.
Please note that scotomatous points measure areas of the retina that have lost all light sensitivity and, therefore, are no longer functioning.
The stock gained 3.3% on Monday, Jun 10, in response to the positive GALE study data. Year to date, Apellis’ shares plunged 30.3% compared with the industry’s 5.9% decline.
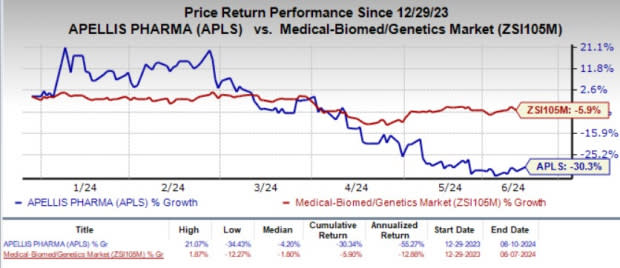
Image Source: Zacks Investment Research
The phase III GALE extension study mostly enrolled patients who completed the late-stage OAKS and DERBY studies, positive results from which supported the approval of Syfovre for the GA indication.
Patients in the sham crossover group completed treatment with sham injections from months 0-24 in the phase III OAKS study and received Syfovre from months 24-36. Microperimetry was a key secondary endpoint measured only in the OAKS study, and therefore, patients who crossed over from the OAKS study were included in the long-term extension study.
Syfovre received FDA approval in February 2023 for the GA indication. It has the potential to be a best-in-class treatment for patients with GA, a disease that is the leading cause of blindness, affecting more than one million people in the United States and five million people worldwide.
The drug has witnessed robust initial uptake since its commercial launch in March 2023.Syfovre recorded sales of $137.5 million in the first quarter of 2024, which increased 20.3% sequentially, owing to continued strong demand, thereby driving revenues for the company. Apellis delivered more than 72,000 commercial vials and nearly 5,000 samples of Syfovre to doctors in the first quarter. As of Mar 31, 2024, the total number of drug doses delivered since launch was 250,000.
A marketing authorization application seeking approval of intravitreal pegcetacoplan to treat GA is currently under review in the EU and several other countries. A decision from the European Medicines Agency is expected no later than July 2024. Approvals from regulatory bodies in other countries are also expected in the current year.Potential approval of the drug in additional geographies will further boost the top line.
Apellis also currently markets pegcetacoplan under the brand name Empaveli/Aspaveli in the United States and EU, as a monotherapy treatment for adult patients suffering from paroxysmal nocturnal hemoglobinuria. The drug is also approved in several other geographies for the same indication.
Apellis Pharmaceuticals, Inc. Price and Consensus
Apellis Pharmaceuticals, Inc. price-consensus-chart | Apellis Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ALX Oncology Holdings ALXO, Annovis Bio ANVS and Compugen CGEN, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73. Year to date, shares of ALXO have plunged 41.8%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
In the past 30 days, the Zacks Consensus Estimate for Annovis’ 2024 loss per share has narrowed from $2.93 to $2.46. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.83 to $1.95. Year to date, shares of ANVS have plunged 65.2%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
In the past 30 days, the Zacks Consensus Estimate for Compugen’s 2024 earnings per share has increased from 2 cents to 5 cents. The consensus estimate for 2025 loss per share is currently pegged at 11 cents. Year to date, shares of CGEN have gained 2.5%.
CGEN’s earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 5.79%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Compugen Ltd. (CGEN) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 