4D Molecular (FDMT) Stock Up 178% in One Month: Here's Why
Shares of 4D Molecular Therapeutics FDMT have skyrocketed 178.3% in the past month against the industry’s 1.5% fall.
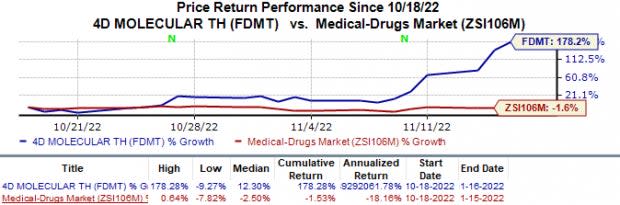
Image Source: Zacks Investment Research
This upside was attributed to positive interim data reported by 4D Molecular from its clinical stage studies on its gene-therapy pipeline candidates in cystic fibrosis and wet age-related macular degeneration (wet AMD) indications.
Last month, FDMT announced interim data from cohort 1 of the phase I/II study evaluating 4D-710 (administered via aerosol delivery) for cystic fibrosis lung disease. Data from the study showed successful widespread delivery and expression of the 4D-710 CFTRΔR transgene in all samples across all study participants. Management intends to report additional data from this study next year. Currently, the company is enrolling study participants in cohort 2 of this phase I/II study.
Earlier this week, 4D Molecular also reported interim data from cohort 1 of the phase I/II PRISM study on intravitreal-administered 4D-150 as a potential treatment for wet AMD. At a low dose of 3E10 vg/eye, patients who received a single intravitreal injection of 4D-150 experienced a clinically significant reduction in annualized anti-VEGF injection rate by 96.7%. Management enrolled participants in the PRISM study who frequently required anti-VEGF injections (i.e., a mean annualized anti-VEGF injection rate of ~11).
This positive interim data also showed that 80% of patients did not require any supplemental aflibercept injections for up to approximately 10 months, thereby remaining anti-VEGF injection-free.
Based on data from the PRISM study, 4D Molecular plans to initiate the phase II expansion portion of the PRISM study in first-quarter 2023. Management also plans to report additional clinical data from all three cohorts of the PRISM study in second-quarter 2023.
Apart from wet AMD, 4D Molecular is planning to evaluate 4D-150 in diabetic macular edema (DME) indication.
During the study, participants treated with 4D-710 or 4D-150 exhibited a well-tolerated safety profile and did not report any adverse events.
Currently, 4D Molecular Therapeutics has no marketed drugs in its pipeline and is entirely dependent on its early-stage gene-therapy candidates for growth. The company’s gene-therapy pipeline also carries significant risks and any negative outcome of any study will affect share prices.
Other than above, the company is also evaluating 4D-125, 4D-110 and 4D-310 in X-linked retinitis pigmentosa ("XLRP"), choroideremia and fabry disease, respectively, in separate phase I/II studies.
4D Molecular Therapeutics, Inc. Price
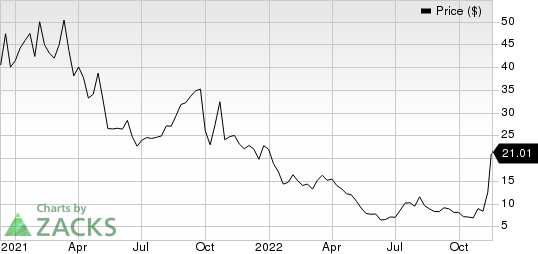
4D Molecular Therapeutics, Inc. price | 4D Molecular Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
4D Molecular currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the overall healthcare sector include Angion Biomedica ANGN, Celularity CELU and Entera Bio ENTX, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Angion Biomedica’s 2022 loss per share have narrowed from $1.64 to $1.53. During the same period, the loss estimates per share for 2023 have narrowed from $1.54 to $1.43. Shares of Angion Biomedica have plunged 70.0% in the year-to-date period.
Earnings of Angion Biomedica beat estimates in three of the last four quarters and missed the mark once, witnessing a surprise of 66.42% on average. In the last reported quarter, ANGN delivered an earnings surprise of 41.67%.
In the past 60 days, estimates for Celularity’s 2022 loss per share have narrowed from 84 cents to 56 cents. During the same period, the loss estimates per share for 2023 have narrowed from $1.04 to $0.97. Shares of Celularity have plunged 63.1% in the year-to-date period.
Earnings of Celularity beat estimates in three of the last four quarters and missed the mark once, witnessing a surprise of 51.01% on average. In the last reported quarter, CELU delivered an earnings surprise of 111.54%.
In the past 60 days, estimates for Entera Bio’s 2022 loss per share have narrowed from 69 cents to 47 cents. During the same period, the loss estimates per share for 2023 have narrowed from 93 cents to 80 cents. Shares of Entera Bio have plunged 77.9% in the year-to-date period.
Earnings of Entera Bio beat estimates in each of the last four quarters, witnessing a surprise of 89.07% on average. In the last reported quarter, ENTX delivered an earnings surprise of 31.25%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Angion Biomedica Corp. (ANGN) : Free Stock Analysis Report
Entera Bio Ltd. (ENTX) : Free Stock Analysis Report
4D Molecular Therapeutics, Inc. (FDMT) : Free Stock Analysis Report
Celularity, Inc. (CELU) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 