A weight loss drug with an illegal ingredient that can cause heart attacks got recalled
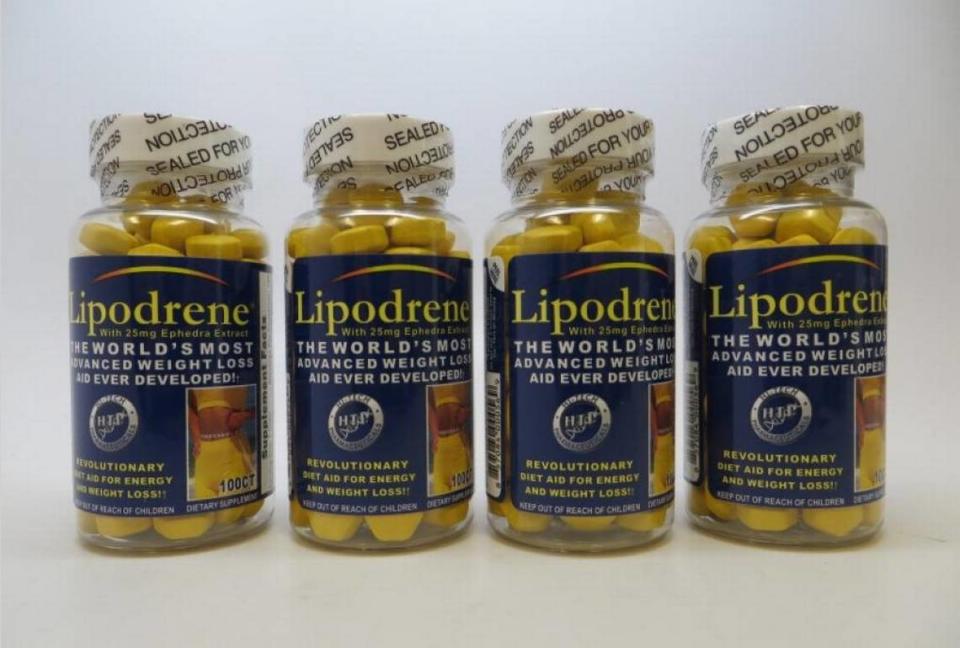
One lot of over-the-counter weight loss drug Lipodrene w/25mg Ephedra Extract Dietary Supplement has been recalled for the presence of an illegal ingredient that the FDA says can cause several serious problems.
“DMAA is not a dietary ingredient, and DMAA-containing products marketed as dietary supplements are illegal and their marketing violates the law,” the FDA said in an August 2018 website post linked off Wednesday’s Lipodrene recall notice.
That notice explains that the FDA’s problem with DMAA (1,4-dimethylamylamine) is that “it can narrow blood vessels and arteries and cause a corresponding rise in blood pressure or other cardiovascular problems, such as: shortness of breath, arrhythmias, elevated blood pressure, tightening in the chest, and heart attack.”
Lipodrene manufacturer Hi-Tech Pharmaceuticals, which wrote the recall notice, says it’s investigating the problem.
This covers lot No. 001211197, expiration date 12/25. The lot number is on the bottom of the bottle. Return the tablets to the retailer or to Hi-Tech for a full refund.
If this or any drug causes a medical problem, notify a medical professional, then let the Food and Drug Administration know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Only then should you consider calling the manufacturer.
To ask questions about this recall, call Hi-Tech at 888-855-7919, 9 a.m. to 5 p.m., Eastern time, or email recallcoordinator@hitechpharma.com.
Problems from pain to cardiac flatline possible after a Pfizer company’s drug mix-up
A thyroid medicine’s third recall: It’s too weak and 43 people had ‘serious’ problems

 Yahoo Finance
Yahoo Finance 