Smart Sock baby monitor that tracks sleep patterns discontinued in U.S. after FDA warning
Owlet Baby Care has discontinued its baby monitor, Smart Sock, in the United States after receiving a warning letter from the U.S. Food & Drug Administration.
The problem: Owlet has been marketing and selling the product — which wraps around a child’s foot and lets parents track their child’s heart rate, oxygen levels and sleep patterns — in the U.S. “without marketing approval, clearance, or authorization” from the FDA.
Smart Socks should also be classified as a medical device, the FDA says, not a “low-risk” general wellness product that promotes a healthy lifestyle. The agency asked Owlet to stop commercial distribution of the device until it addresses the violations or risk possible regulatory actions including seizure, injunction and monetary penalties.
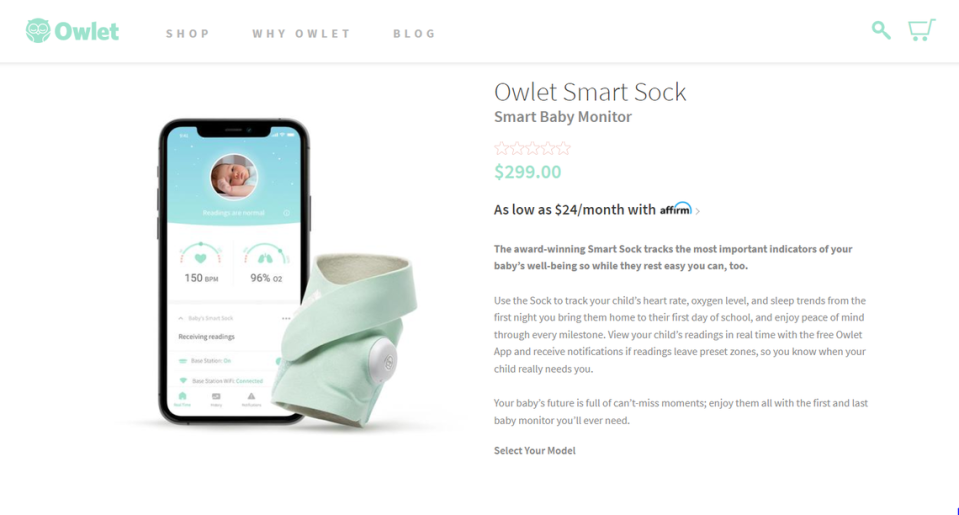
Owlet’s decision to stop U.S. sales of its Smart Sock product ends a lengthy tug-of-war, at least for now, between the Los Angeles-based company and the federal government that goes back to 2016. Owlet announced the discontinuation of its Smart Socks this week and no longer has the product on its website.
Smart Socks were being sold at retailers including Target, Walmart, Kohl’s, and Bed Bath & Beyond, as well as Amazon. As of Thursday afternoon, it appeared some websites were still letting you purchase one.
“The letter we received from the agency did not identify any safety concerns about the Smart Sock,” Owlet said in a statement posted on its website. The company said it has sold more than one million Smart Socks and that the device “has been validated by third parties, in which it was shown to be safe.”
Owlet also said it plans to submit a device application to the FDA for the Smart Sock and noted that the agency had not requested any returns or exchanges of the product.
The company also said its pause in Smart Sock sales will only apply in the U.S. and that it’s also planning to launch a new baby monitoring system, the Dream Sock, in the U.S. in January.

 Yahoo Finance
Yahoo Finance 