Shockwave Medical (SWAV) Launches IVL Catheter in Select Markets
Shockwave Medical, Inc. SWAV recently announced the full commercial availability of the Shockwave C2+ Coronary Intravascular Lithotripsy (IVL) Catheter. The device is used for the treatment of severely calcified coronary artery disease (CAD) in select international markets.
Shockwave C2+ is commercially available for treating de novo CAD in Europe and select other geographies.
In the same press release, Shockwave Medical also announced the enrollment of the first patient in EMPOWER CAD, the first prospective, all-female study of percutaneous coronary intervention. The study seeks to confirm the benefits of coronary IVL in female patients with calcified lesions, who historically have experienced less favorable clinical outcomes than male patients with traditional therapies.
The latest commercial availability is expected to aid Shockwave Medical in significantly expanding its footprint in the CAD treatment space and solidifying its position worldwide. The enrollment of patients in the study is also a major stepping stone for the company toward achieving its goal.
Significance of the Launch and Enrollment
Per an expert familiar with the use of the Shockwave C2+ Catheter, its intuitive catheter design and ease of use incorporate improvements that will likely enhance procedural efficiency and optimize the treatment of the most challenging morphologies.
With respect to the enrollment of patients in the EMPOWER CAD study, an expert associated with it believes the study to be a major step toward better understanding the optimal strategy for calcium modification in female patients. Per the personnel, females are an under-represented patient population, who are often more challenging to treat and experience sub-optimal outcomes.
Industry Prospects
Per a report by Research and Markets published on yahoo! finance, the global CAD market is expected to reach $38.17 billion in 2028 from $22.94 billion in 2022 at a CAGR of approximately 8.9%. Factors like rapid growth in the elderly population, unhealthy lifestyles and a rising number of people suffering from obesity, diabetes and high blood pressure are likely to drive the market.
Given the market potential, the latest commercial availability is expected to provide a significant boost to Shockwave Medical’s business globally.
Notable Developments
This month, Shockwave Medical announced its first-quarter 2023 results, wherein it registered a solid uptick in its overall top line. Per management, the strong growth in the top line was driven by double-digit growth in the U.S. coronary, U.S. peripheral and International markets. Key revenue drivers include strong uptake of M5+ catheter, the addition of new accounts and appropriate reimbursement. Internationally, SWAV’s sales were boosted by strong momentum in Germany and Japan and a robust contribution from China.
Last month, Shockwave Medical announced the completion of its previously-announced acquisition of Neovasc Inc.
In March, Shockwave Medical announced the full U.S. commercial availability of the Shockwave L6 Peripheral IVL Catheter. This follows the receipt of the FDA’s clearance for the same.
Price Performance
Shares of the company have gained 68.3% in the past year compared with the industry’s 1.9% rise and the S&P 500's 1.3% growth.
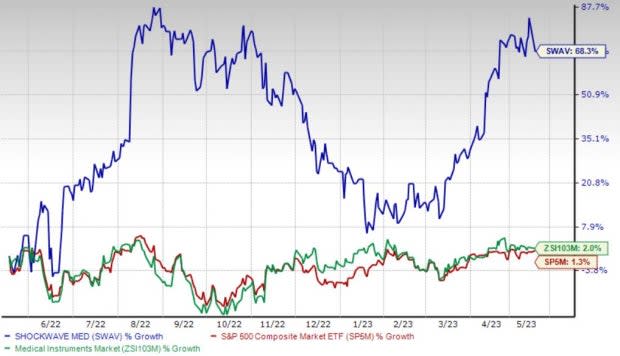
Image Source: Zacks Investment Research
Zacks Rank & Key Picks
Currently, Shockwave Medical carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are AmerisourceBergen Corporation ABC, Merit Medical Systems, Inc. MMSI and DaVita Inc. DVA.
AmerisourceBergen, carrying a Zacks Rank #2 (Buy) at present, has an estimated long-term growth rate of 8.9%. ABC’s earnings surpassed estimates in all the trailing four quarters, the average being 3.1%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
AmerisourceBergen has gained 10.5% compared with the industry’s 7.4% rise in the past year.
Merit Medical, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 20.2%.
Merit Medical has gained 35.2% compared with the industry’s 7.4% rise over the past year.
DaVita, carrying a Zacks Rank #2 at present, has a long-term estimated growth rate of 14.6%. DVA’s earnings surpassed estimates in three of the trailing four quarters and missed in one, the average surprise being 17.3%.
DaVita has lost 1.9% compared with the industry’s 18% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
DaVita Inc. (DVA) : Free Stock Analysis Report
AmerisourceBergen Corporation (ABC) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report
ShockWave Medical, Inc. (SWAV) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 