Pharma Stock Roundup: PFE's Biohaven Buyout Offer, BAYRY's Q1 Earnings Update
This week, Pfizer PFE offered to buy migraine drugmaker, Biohaven Pharmaceuticals for $11.6 billion. Bayer BAYRY announced first-quarter 2022 earnings. Roche RHHBY announced disappointing data from a phase III PD-L1-high metastatic non-small cell lung cancer (NSCLC)study. The FDA granted full approval to Eli Lilly LLY and Incyte’s Olumiant for treating COVID-19 in certain hospitalized patients. The drug has been available in the United States under emergency approval since 2020. AbbVie’s ABBV Rinvoq achieved clinical remission and endoscopic response at one year in a phase III maintenance study in patients with Crohn's disease.
Recap of the Week’s Most Important Stories
Pfizer to Buy Biohaven for $11.6B: Pfizer announced a definitive agreement to acquire Biohaven for a purchase consideration of approximately $11.6 billion in cash. The acquisition will add the latter’s migraine therapy, rimegepant, which is approved as Nurtec ODT in the United States for both acute treatment and episodic prevention of migraine in adults.
Rimegepant was recently approved as Vydura in the European Union for both acute treatment of migraine and prophylaxis of episodic migraine. Biohaven’s calcitonin gene-related peptide (CGRP) also includes zavegepant for which a regulatory application was filed in the United States in March as an intranasal spray for the acute treatment of migraine. Pfizer already has commercialization rights to rimegepant and zavegepant outside U.S. markets, per a deal signed in November last year as well as a 2.6% stake in Biohaven.
Per the deal, Pfizer will acquire all outstanding shares of Biohaven that it does not already own for $148.50 per share in cash. Biohaven shareholders will also get 0.5 of a share of a new publicly-traded company that will retain Biohaven’s non-CGRP pipeline compounds. The new company will continue to operate under the Biohaven name. Pfizer’s acquisition of Biohaven is expected to close in early 2023.
Pfizer and partner Myovant Sciences announced that the FDA has extended the review period of Myovant Sciences’ supplemental new drug application (sNDA) for Myfembree by three months.
This sNDA seeks approval for Myfembree (relugolix 40 mg, estradiol 1 mg and norethindrone acetate 0.5 mg) to manage moderate-to-severe pain associated with endometriosis. The FDA delayed its decision to Aug 6 from May 6 as it needs time to review new information submitted by the companies on FDA’s request regarding bone mineral density.
Last month, the companies had received a notice from the FDA that identified deficiencies that precluded the decision on labeling and/or post-marketing requirements in the sNDA
Bayer Tops Q1 Earnings & Sales Estimates: Bayer beat estimates for both earnings and sales in the first quarter. While sales in the Crop Science unit rose 21.6%, Pharmaceutical unit sales rose 2.6% year over year. Sales of key drug, Eylea rose 13.9%, driven by strong growth as a result of high demand in Europe and China. Sales in the Consumer Health segment rose 17.2%, supported by a robust recovery from the pandemic across all regions. The company maintained its previously issued core earnings guidance of €7 per share and sales outlook of €46 billion in 2022.
Roche’s Lung Cancer Study Fails to Improve PFS:Roche’s phase III study evaluating its investigational anti-TIGIT immunotherapy tiragolumab plus its PD-L1 inhibitor Tecentriq for the first-line treatment of PD-L1-high metastatic non-small cell lung cancer (NSCLC) failed to meet its co-primary endpoint of progression-free survival. The second primary endpoint of overall survival was not mature and the SKYSCRAPER-01 study will continue for further analysis. However, the study did achieve a numerical improvement for both the primary endpoints.
FDA’s Full Approval to Lilly’s Olumiant for Hospitalized COVID-19: The FDA granted full approval to Lilly/Incyte’s JAK inhibitor Olumiant (baricitinib) for treating COVID-19 in certain hospitalized adults requiring various degrees of oxygen support. Olumiant is already authorized for emergency use for this indication by the FDA since November 2020. However, the FDA’s full approval comes with a boxed warning on its label about the risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE) and thrombosis. Olumiant is presently approved to treat rheumatoid arthritis (RA) in several countries and authorized to treat hospitalized COVID-19 patients in approximately 15 countries. Olumiant is also approved in Europe and Japan for atopic dermatitis while it is under review in the United States for the same indication.
AbbVie’s Rinvoq Meets Goal in Crohn’s Disease Study: AbbVie’s U-ENDURE, a phase III maintenance study evaluating Rinvoq (upadacitinib) in adult patients with moderate to severe Crohn's disease met the primary endpoint. In the study, a significantly greater proportion of patients treated with either 15 mg or 30 mg of upadacitinib achieved the co-primary endpoints of clinical remission and endoscopic response and a key secondary endpoint of endoscopic remission at one year compared to placebo. Rinvoq has been studied as an oral medicine for moderate-to-severe Crohn's disease in two other studies as well, U-EXCEED and U-EXCEL induction studies.
The NYSE ARCA Pharmaceutical Index declined 0.9% in the last five trading sessions.
Here’s how the eight major stocks performed in the last five trading sessions.
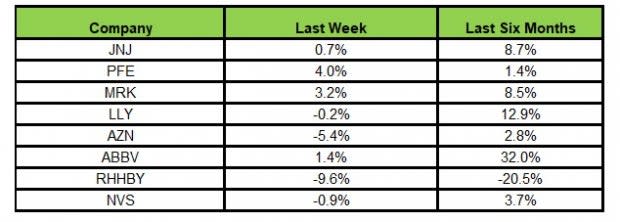
Image Source: Zacks Investment Research
In the last five trading sessions, Pfizer rose the most (4%) while Roche declined the most (9.6%).
In the past six months, AbbVie rose the most (32%) while Roche declined the most (20.5%).
(See the last pharma stock roundup here: Q1 Earnings of LLY, MRK & NVS, FDA Updates for AZN & PFE)
What's Next in the Pharma World?
Watch for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 