Pharma Stock Roundup: FDA Approval for NVO, PFE Products & Other Updates
This week the FDA approved Novo Nordisk’s NVO GLP-1 product, semaglutide, to be marketed as Wegovy, for weight loss in people living with obesity and Pfizer’s PFE 20-valent pneumococcal conjugate vaccine, Prevnar 20. Merck MRK signed a deal with the U.S. government to supply 1.7 billion doses of its COVID-19 candidate molnupiravir, if it gets approved by the FDA.
Recap of the Week’s Most Important Stories
FDA Approves Novo Nordisk’s Obesity Drug Wegovy: The FDA approved Novo Nordisk’s GLP-1 product, semaglutide as a weekly 2.4 mg injection for weight management in people living with obesity, to be marketed by the brand name of Wegovy. The approval was based on data from the phase IIIa STEP study, which enrolled around 4,500 adults with overweight or obesity. Data from the study showed that people with obesity and without type II diabetes, experienced an average weight loss of 17-18% sustained over 68 weeks when given Wegovy.
FDA Approves Pfizer’s Pneumococcal Vaccine: The FDA also granted approval to Pfizer’s 20-valent pneumococcal conjugate vaccine, Prevnar 20, for the prevention of invasive disease and pneumonia in adults. Prevnar 20 includes all the 13 serotypes contained in Pfizer’s popular 13-valent pneumococcal conjugate vaccine, Prevnar 13 along with seven additional serotypes. The vaccine helps protect against 20 serotypes responsible for the majority of invasive pneumococcal disease and pneumonia.
Meanwhile, Pfizer and partner BioNTech BNTX have agreed to provide 500 million doses of their COVID-19 vaccine to U.S. government at a not-for-profit price to donate to 100 low- and lower middle-income countries. Of the 500 million doses, 200 million will be supplied in 2021 and 300 million doses in the first half of 2022. Pfizer and BioNTech had earlier pledged to provide two billion doses of the COVID-19 vaccine to poor nations. These 500 million doses are part of this pledge.
Merck’s COVID Candidate Supply Deal With U.S. Government: Merck announced a supply deal with the U.S. government for molnupiravir, its investigational oral antiviral for COVID-19. The government has committed to buy approximately 1.7 million courses of molnupiravir, once it gets emergency approval from the FDA. Merck is developing molnupiravir in partnership with Ridgeback Biotherapeutics in a phase III study for the treatment of non-hospitalized patients with confirmed COVID-19. For the deal, Merck will receive approximately $1.2 billion.
Novartis’s Phase II Data on Rare Kidney Disease Candidate: Novartis’ NVS phase II study evaluating iptacopan (LNP023), a first-in-class, oral, targeted factor B inhibitor for rare kidney disease IgA nephropathy (IgAN), met its primary endpoint. The candidate reduced protein in the urine (proteinuria), an increasingly recognized surrogate marker correlating with progression to kidney failure, and showed immense promise in stabilizing kidney function among patients with IgAN. IgAN currently does not have approved treatments.
The NYSE ARCA Pharmaceutical Index rose 4.3% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return

Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
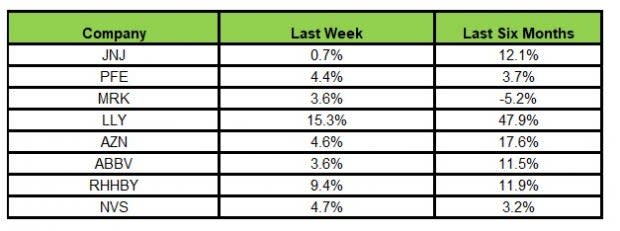
Image Source: Zacks Investment Research
In the last five trading sessions, all the stocks were in the green. Lilly LLY rose the most (15.3%).
In the past six months, Lilly has recorded the maximum gain (47.9%) while Merck declined the most (5.2%)
(See the last pharma stock roundup here: JNJ Cancer Drug Gets FDA Nod, PFE, GSK, SNY Begin New Studies)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
+1,500% Growth: One of 2021’s Most Exciting Investment Opportunities
In addition to the stocks you read about above, would you like to see Zacks’ top picks to capitalize on the Internet of Things (IoT)? It is one of the fastest-growing technologies in history, with an estimated 77 billion devices to be connected by 2025. That works out to 127 new devices per second.
Zacks has released a special report to help you capitalize on the Internet of Things’s exponential growth. It reveals 4 under-the-radar stocks that could be some of the most profitable holdings in your portfolio in 2021 and beyond.
Click here to download this report FREE >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Novo Nordisk AS (NVO) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 