Pharma Stock Roundup: End of SNY's Amcenestrant Development, FDA Updates for AZN, GSK
This week, Sanofi SNY discontinued the development of its oral SERD, amcenestrant while Novartis’ NVS lung cancer candidate, canakinumab missed the primary endpoint in a phase III study. The FDA granted priority review status to AstraZeneca AZN and Merck’s MRK Lynparza as a combination therapy for patients with metastatic castration-resistant prostate cancerand accepted GSK’s GSK regulatory application seeking approval for momelotinib for treating myelofibrosis.
Recap of the Week’s Most Important Stories
Sanofi Discontinues Development of Amcenestrant: Sanofi announced that it is discontinuing the development of its breast cancer candidate, amcenestrant, following the failure of a second study, AMEERA-5. The AMEERA-5 phase III study was evaluating amcenestrant, an oral SERD, in combination with Pfizer’s Ibrance (Palbociclib) compared with letrozole plus Ibrance in patients with ER+/HER2- advanced breast cancer. An Independent Data Monitoring Committee (IDMC) recommended stopping the study after the amcenestrant combination failed to meet the pre-specified boundary for continuation in comparison with the control arm. Earlier, in March, a phase II study (AMEERA-3) on amcenestrant for ER+/HER2- advanced or metastatic breast cancer failed to meet its primary endpoint of improving progression-free survival (PFS) as assessed by an independent central review. All clinical studies on amcenestrant have been discontinued.
Novartis’ NSCLC Study on Canakinumab Fails: Novartis’ phase III lung cancer study on IL-1β inhibitor candidate, canakinumab failed to meet its primary endpoint of disease-free survival (DFS) versus placebo. The CANOPY-A study evaluated canakinumab (ACZ885) as an adjuvant treatment for adult patients with stages II-IIIA and IIIB completely resected (R0) non-small cell lung cancer (NSCLC). Earlier, in the phase III cardiovascular CANTOS study, canakinumab led to significantly lower-than-expected rates of lung cancer mortality among patients. After observing the positive results of the CANTOS study, Novartis launched the CANOPY study program. Canakinumab is already approved under the brand name Ilaris for arthritis, among others.
FDA’s Priority Tag to AstraZeneca’s Enhertu for Expanded Use in Prostate Cancer: The FDA granted priority review to a supplemental new drug application (sNDA) seeking approval for AstraZeneca and Merck’s Lynparza in combination with J&J’s prostate cancer drug, Zytiga (abiraterone), and corticosteroid prednisone as a treatment for metastatic castration-resistant prostate cancer (mCRPC). With the FDA granting priority review to the sNDA, a decision is expected in the fourth quarter of 2022. The sNDA filing was based on data from the phase III study — PROpel. In prostate cancer, Lynparza is presently approved for treating HRR gene-mutated mCRPC (BRCA-mutated and other HRR gene mutations) in the United States.
AstraZeneca’s phase III study, DESTINY-Breast02, evaluating it and partner Daiichi Sankyo’s drug Enhertu in previously treated HER2-positive metastatic breast cancer met the primary endpoint, demonstrating a statistically significant and clinically meaningful improvement in progression-free survival compared to physician’s choice of treatment. The study also met the key secondary endpoint of improved overall survival.
Merck’s New RNA-Based Collaboration Deal: Merck entered into a collaboration deal with privately held Orna Therapeutics to discover and develop vaccines and medicines for infectious disease and oncology, leveraging Orna’s next generation of RNA technologycalled circular RNA (oRNA) technology. Orna believes that its circular RNA technology will be more effective than traditional, linear RNA technology in treating diseases as they have greater stability as well as the potential to produce larger quantities of therapeutic proteins inside the body.For the deal, Merck will make an upfront payment of $150 million to Orna. In addition, Merck will make $3.5 billion in milestone payments to Orna. In addition, Merck will invest $100 million in Orna’s equity.
FDA Accepts GSK’s Momelotinib NDA: The FDA accepted GSK’s NDA seeking approval for momelotinib for treating myelofibrosis, a type of bone marrow cancer. The FDA decision on the NDAis expected on Jun 16, 2023. Momelotinib was added to GSK’s hematology portfolio with the July acquisition of California-based cancer biotech, Sierra Oncology. The NDA filing was based on data from key phase III studies, including the pivotal MOMENTUM study. In the MOMENTUM study, momelotinib achieved statistical significance in the primary and all pre-specified secondary endpoints.
The NYSE ARCA Pharmaceutical Index declined 0.04% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return

Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
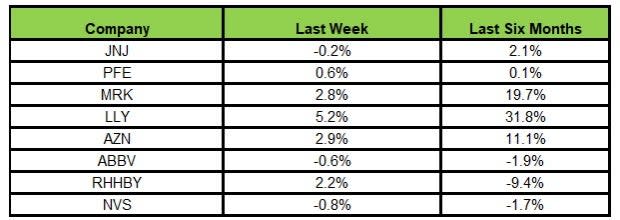
Image Source: Zacks Investment Research
In the last five trading sessions, Lilly rose the most (5.2%) while Novartis declined the most (0.8%).
In the past six months, Lilly rose the most (31.8%) while Roche declined the most (9.4%).
(See the last pharma stock roundup here: PFE to Buy GBT, JNJ to Stall Baby Talc Sale Globally From 2023)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 