Pfizer (PFE) Seeks FDA Approval for Omicron Booster in Kids
Pfizer, Inc. PFE and its partner BioNTech BNTX have submitted a regulatory filing to the FDA, seeking to expand the emergency use authorization (EUA) granted to their bivalent BA.4/BA.5 Omicron-targeting COVID-19 vaccine.
This regulatory filing seeks label expansion for using the 10-µg booster dose of Pfizer/BioNTech’s bivalent COVID-19 vaccine in children aged five through 11 years. Currently, a 30-µg booster dose of this vaccine is authorized for usein individuals aged 12 years and older.
The bivalent vaccine contains 15-µg of an mRNA encoding the spike protein present in the original vaccine and 15 µg of an mRNA encoding the spike protein that is common in the Omicron BA.4 and BA.5 variants.
Pfizer/BioNTech’s filing seeking authorization for using the Omicron BA.4/BA.5 booster in kids is based on clinical studies on their bivalent Omicron BA.1-adapted vaccine as well as pre-clinical data from studies on their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine. However, unlike all previously-approved COVID-19 vaccines, the data on the bivalent booster shot is not supported by data from any human clinical studies demonstrating the vaccine’s effectiveness.
Pfizer/BioNTech have initiated clinical studies on the Omicron BA.4/BA.5-adapted bivalent vaccine, though the results will not be available before some months.
Shares of Pfizer have lost 25.8% in the year so far compared with the industry’s 5.6% decline.
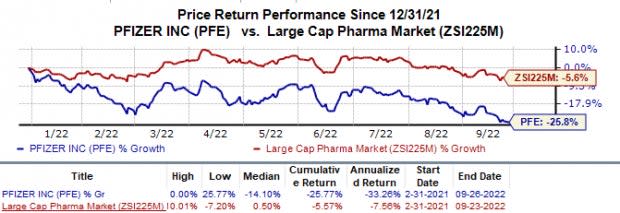
Image Source: Zacks Investment Research
Pfizer/BioNTech also submitted a regulatory filing to the European Medicines Agency (“EMA”) seeking conditional marketing authorization (cMA) for their Omicron BA.4/BA.5-adapted bivalent vaccine in individuals aged 12 years and older. Earlier this month, the EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended approving the cMA for the bivalent vaccine. Pfizer/BioNTech also plans to submit an application to the EMA in the coming days to expand the cMA to include children aged 5 through 11 years.
Last month, the FDA granted EUA to Pfizer/BioNTech and Moderna’s MRNA Omicron BA.4/BA.5-adapted bivalent vaccines for a single booster dose. The Pfizer/BioNTech and Moderna booster vaccines can be given at least two months following primary or booster vaccination. While Moderna’s Omicron booster is authorized for use in adults aged 18 years and older, Pfizer/BioNTech’s version is approved for people 12 and older.
These booster vaccines were developed at the FDA’s recommendation, issued back in June, to modify the design of their respective vaccine boosters to target the Omicron BA.4 and BA.5 subvariants.
Pfizer Inc. Price
Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank & Stocks to Consider
Pfizer currently carries a Zacks Rank #3 (Hold).A better-ranked stock in the overall healthcare sector is Morphic MORF, which sports a Zacks Rank #1 (Strong Buy).You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Morphic’s 2022 loss per share have narrowed from $3.38 to $1.80. Loss estimates for 2023 have narrowed from $3.91 to $3.62 during the same period. Shares of Morphic have lost 46.7% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 48.29%, on average. In the last reported quarter, MORF delivered an earnings surprise of 183.95%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 