OPKO's (OPK) NGENLA Approved by Japan's MHLW for GHD Treatment
OPKO Health, Inc. OPK, along with Pfizer Japan Inc., a subsidiary of Pfizer Inc. PFE, recently confirmed that Japan’s Ministry of Health, Labour and Welfare (“MHLW”) has approved NGENLA (somatrogon) Inj. 24 mg Pens and 60 mg Pens. NGENLA, a next-generation long-acting growth hormone injection, is a once-weekly long-acting recombinant human growth hormone. It is approved for the indication of short stature resulting from growth hormone deficiency (“GHD”) without closed epiphyses (another term for pineal gland).
OPKO had entered into a worldwide agreement with Pfizer in 2014 for the development and commercialization of somatrogon for the treatment of GHD. According to agreement, OPKO had to conduct the clinical program while Pfizer was responsible for registering and commercializing somatrogon for GHD.
The latest approval for NGENLA in Japan follows its approval in Canada in October 2021 and in Australia in November 2021. Japan’s MHLW’s approval is expected to significantly strengthen OPKO’s position through the global sales of NGENLA, a component of the company’s broader Pharmaceuticals business.
Background of the Approval
The approval by Japan’s MHLW was backed by the results of a Phase 3 study conducted on Japanese subjects and a global Phase 3 clinical study. Both studies were conducted on subjects with pediatric GHD, which compared the efficacy and safety of once-weekly NGENLA with GENOTROPIN (somatropin) — a recombinant human growth hormone for injection, administered once daily.
Both studies demonstrated the comparable efficacy of NGENLA to GENOTROPIN in the primary endpoint of annual height growth rate at 12 months. Also, unlike GENOTROPIN, NGENLA was generally well endured in both studies with comparable safety.
Significance of the Approval
NGENLA, a new option patients with pediatric GHD, lowers treatment frequency from daily to once-weekly injections.
Per Pfizer’s Japanese management, the recent approval for once-weekly NGENLA is expected to lead to a reduction in the burden related to daily growth hormone administration.
Industry Prospects
Per a report by Allied Market Research, the global human growth hormone market was valued at $3,864.00 million in 2020 and is projected to reach $9,211.63 million by 2030 at a CAGR of 9%. Factors like the development of recombinant human growth hormone drugs, rise in pituitary dysfunction cases, and increasing cases of growth hormone deficiency and other growth disorders are expected to drive the market.
Given the market potential, the latest approval is expected to significantly boost OPKO’s global Pharmaceuticals business.
Recent Updates
This month, OPKO signed a definitive agreement with AI-driven genomic and clinical data intelligence platform company, Sema4. Per the terms of the agreement, Sema4 will acquire OPKO’s wholly owned subsidiary, GeneDx, Inc.
In December 2021, the company announced preliminary top-line results from its Phase 2 trial with RAYALDEE for the treatment of mild-to-moderate COVID-19.
Also in December, OPKO received the FDA’s approval for its 4Kscore Test. The test is approved for use in men aged 45 and above who have not had a prior prostate biopsy or are biopsy negative, and have an age-specific abnormal total prostate specific antigen and/or abnormal digital rectal exam.
Price Performance
Shares of OPKO have lost 1.1% in the past year compared with the industry’s 6.9% fall. The S&P 500 has risen 18.4% in the same time frame.
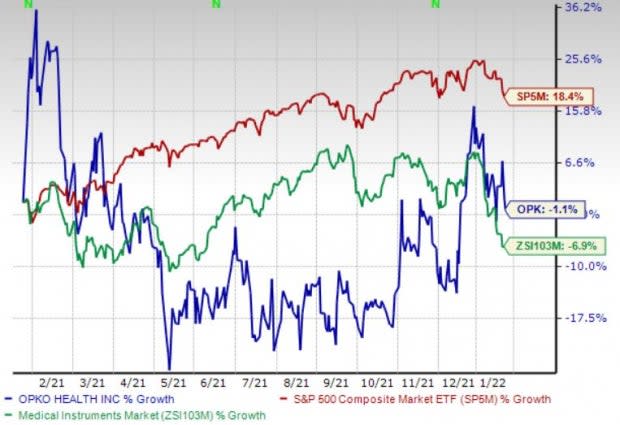
Image Source: Zacks Investment Research
Zacks Rank & Other Key Picks
Currently, OPKO carries a Zacks Rank #2 (Buy).
A couple of other stocks from the broader medical space that investors can consider are AMN Healthcare Services, Inc. AMN and Cerner Corporation CERN.
AMN Healthcare has an estimated long-term growth rate of 16.2%. AMN’s earnings surpassed estimates in the trailing four quarters, the average surprise being 19.51%. It currently flaunts a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
AMN Healthcare has gained 28.7% against the industry’s 60.3% fall over the past year.
Cerner, carrying a Zacks Rank #2, has an estimated long-term growth rate of 12.8%. CERN’s earnings surpassed estimates in three of the trailing four quarters, the average surprise being 3.21%.
Cerner has gained 14.5% against the industry’s 56.3% fall over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Cerner Corporation (CERN) : Free Stock Analysis Report
AMN Healthcare Services Inc (AMN) : Free Stock Analysis Report
OPKO Health, Inc. (OPK) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 