Myriad Genetics' (MYGN) New Pact to Extend HRD Testing Access
Myriad Genetics, Inc. MYGN recently announced the expansion of a strategic partnership with Illumina Inc. ILMN to widen access to oncology homologous recombination deficiency (HRD) testing in the United States. Per the agreement, Illumina’s TruSight Oncology 500 HRD (TSO 500 HRD) — a research-use-only test — is now obtainable in the United States.
The latest development is expected to significantly boost Myriad Genetics’ Oncology business, which is a part of the broader Molecular Diagnostic Testing segment.
About TruSight Oncology 500 and TSO 500 HRD
TSO 500 HRD offers a comprehensive pan-cancer test to identify key genetic variants and homologous recombination deficiency critical for understanding cancer development and progression. In addition, it evaluates key genomic signatures, such as tumor mutational burden, microsatellite instability and HRD.
The TSO 500 HRD research test aligns with Myriad’s MyChoice CDx HRD technology with Illumina’s pan-cancer test, TSO 500. The test was co-developed with Merck (known as MSD outside the US and Canada) and Myriad Genetics.
More on Partnership
Under this strategic partnership, Myriad Genetics and Illumina will seek joint HRD companion diagnostics partnerships with pharmaceutical companies globally (excluding Japan). The joint HRD CDx partnership will aim to follow HRD regulatory approvals for both the MyChoice HRD Assay companion diagnostic and a future clinical in vitro diagnostic test based on the TSO 500 HRD Assay.
The expanded partnership also establishes a unique companion diagnostic (CDx) alliance for the pharmaceutical industry, which will enable more clinical research for gene-based, targeted therapies.
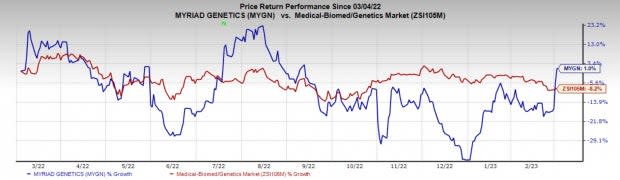
Image Source: Zacks Investment Research
With the partnership, the product is now available to order and ready to ship in the United States. Both companies provide distributable kits and centralized laboratory services, respectively.
Strategic Implications
Per Myriad Genetics’ management, the partnership with Illumina brings together the best quality HRD technology and latest-generation sequencing to develop a comprehensive testing solution that aids the advancement of clinical research leading to enhanced patient outcomes.
Management also noted that the availability of TSO 500 HRD in the United States strengthens Myriad Genetics’ ability to partner with leading pharmaceutical companies and academic institutions, broadens access to clinical trials and advances the pace of research and scientific innovation.
This CDx alliance aims to further allow clinical research for HRD testing and therapeutics, worldwide, which could lead to more access to clinical trials for precision, gene-based therapies.
Industry Prospects
Per a report by Grand View Research, the global cancer diagnostics market size was valued at $160.5 billion in 2021 and is expected to expand at a CAGR of 6.89% by 2030. The increasing number of cancer cases globally and technological advancements in diagnostic tests drive the market.
Recent Developments
In November 2022, Myriad Genetics launched UroSuite, which is a comprehensive suite of genetic risk assessment tests that cover every stage of prostate cancer care. The UroSuite includes the company’s Prolaris Prostate Cancer Test, MyRisk Hereditary Cancer Test, BRACAnalysis CDx and Precise Tumor Molecular Profile Test.
In the same month, Myriad Genetics announced that JCO Precision Oncology had published a study highlighting the development and validation of a breast cancer polygenic risk score for women of all ancestries.
Price Performance
Shares of the company have gained 1% in the past year against the industry’s fall of 8.2%.
Zacks Rank and Key Picks
Currently, Myriad Genetics carries a Zacks Rank #3 (Hold).
A couple of other top-ranked stocks in the overall healthcare sector include Haemonetics Corporation HAE, TerrAscend Corp. TRSSF, both sport a Zacks Rank #1(Strong Buy), You can see the complete list of today’s Zacks #1 Rank stocks here.
Haemonetics’ stock has risen 42.1% in the past year. Earnings Estimates for Haemonetics have increased from $2.87 per share to 2.91 for 2023 and from $3.02 per share to $3.28 for 2024 in the past 30 days.
HAE’s earnings beat estimates in each of the last four quarters, delivering an average surprise of 10.98%. In the last reported quarter, it reported an earnings surprise of 7.59%.
Estimates for TerrAscend in 2023 have remained constant at a loss of 10 cents per share in the past 30 days. Shares of TerrAscend have declined 70.6% in the past year.
TerrAscend’s earnings beat estimates in one of the last three quarters and missed the mark in the other two, the average negative surprise being 136.11%. In the last reported quarter, TRSSF delivered an earnings surprise of 216.67%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Illumina, Inc. (ILMN) : Free Stock Analysis Report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
Myriad Genetics, Inc. (MYGN) : Free Stock Analysis Report
TerrAscend Corp. (TRSSF) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 