Minerva (NERV) Down; FDA Denies Review Schizophrenia Drug NDA
Shares of Minerva Neurosciences NERV were down 38.9% on Dec 28 after management announced that the FDA’s refuse-to-file letter on the company’s new drug application (“NDA”) filing was still in effect. The NDA filing sought approval for roluperidone to treat negative symptoms in patients with schizophrenia.
A refusal-to-file letter is issued by FDA when it refuses to accept a marketing application due to significant deficiencies in the application that the regulatory body believe cannot be promptly resolved.
The FDA issued the refusal-to-file letter on Minerva’s NDA on Oct 14, 2022. This letter is still in force despite a Type A meeting requested by Minerva with the FDA, which was subsequently held on Nov 30, 2022. The company will continue coordinating with FDA officials to evaluate the next steps for roluperidone.
Investors were unimpressed with the announcement as roluperidone is the only late-stage candidate in company’s pipeline. Apart from roluperidone, Minerva is evaluating MIN-301, an early-stage pipeline candidate being developed as a potential treatment for Parkinson’s disease.
Neither the FDA nor Minerva disclosed the deficiencies in the NDA for which the refuse-to-file letter was issued.
Shares of Minerva have lost 77.7% in the year compared with the industry’s 31.0% decline.
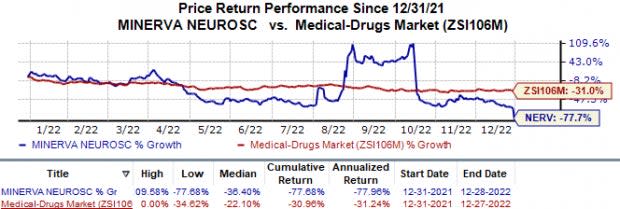
Image Source: Zacks Investment Research
Minerva’s NDA for roluperidone is supported by data from two clinical studies — a phase IIb study and phase III study — which evaluated the drug in schizophrenia patients with negative symptoms. Management believes that data from the studies are sufficient to support the long-term safety and efficacy of adult patients with schizophrenia.
Per Minerva, negative symptoms in schizophrenia are the main cause of poor functional outcomes and are also likely one of the main reasons ultra-high-risk adolescents develop full-blown schizophrenia. Currently, there are no FDA-approved therapies for treating negative symptoms of schizophrenia.
Minerva Neurosciences, Inc Price

Minerva Neurosciences, Inc price | Minerva Neurosciences, Inc Quote
Zacks Rank & Stocks to Consider
Minerva currently carries a Zacks Rank #4 (Sell). Some better-ranked stocks in the overall healthcare sector include Acer Therapeutics ACER, Amylyx Pharmaceuticals AMLX and Can-Fite BioPharma CANF. Acer Therapeutics sports a Zacks Rank #1 (Strong Buy) at present, while Amylyx Pharmaceuticals and Can-Fite BioPharma carry a Zacks Rank #2 *Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Acer Therapeutics’ 2022 loss per share have narrowed from $2.47 to $1.50. During the same period, the loss estimates per share for 2023 have narrowed from $1.07 to 61 cents. Shares of Acer Therapeutics have risen 12.7% year to date.
Earnings of Acer Therapeutics beat estimates in two of the last four quarters and missed the mark twice, witnessing a negative earnings surprise of 95.82%, on average. In the last reported quarterAcer Therapeutics’ earnings beat estimates by 59.21%.
In the past 60 days, estimates for Amylyx Pharmaceuticals’ 2022 loss per share have narrowed from $3.54 to $3.53. During the same period, the loss estimates per share for 2023 have narrowed from 65 cents to 60 cents. Shares of Amylyx Pharmaceuticals have surged 93.4% in the year-to-date period.
Earnings of Amylyx Pharmaceuticals missed estimates in two of the last three quarters and beat the mark once, witnessing a negative earnings surprise of 6.65%, on average. In the last reported quarter, Amylyx Pharmaceuticals’ earnings beat estimates by 5.15%.
In the past 60 days, estimates for Can-Fite BioPharma’s 2022 loss per share have narrowed from 37 cents to 28 cents. During the same period, the loss estimates per share for 2023 have narrowed from 17 cents to 12 cents. Shares of Can-Fite BioPharma have declined 49.7% in the year-to-date period.
Earnings of Can-Fite BioPharma beat estimates in three of the last four quarters and missed the mark once, witnessing an earnings surprise of 10.14%, on average. In the last reported quarter, Can-Fite BioPharma’s earnings beat estimates by 10.00%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
CanFite Biopharma Ltd (CANF) : Free Stock Analysis Report
Minerva Neurosciences, Inc (NERV) : Free Stock Analysis Report
Amylyx Pharmaceuticals, Inc. (AMLX) : Free Stock Analysis Report
Acer Therapeutics Inc. (ACER) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 