Merck (MRK) Keytruda Advanced Gastric Cancer Study Meets Goal
Merck MRK announced positive data from the phase III KEYNOTE-859 study evaluating its blockbuster anti-PD-1 therapy drug, Keytruda. The drug is targeted toward first-line treatment of HER2-negative locally advanced unresectable or metastatic gastric or Gastroesophageal Junction (GEJ) adenocarcinoma. The study achieved its primary endpoint of overall survival (OS).
Data from the study showed that the combination of Keytruda and chemotherapy exhibited a statistical and clinical improvement in OS in study participants, regardless of PD-L1 expression, compared with those treated with chemotherapy alone. Patients who were administered Keytruda plus chemotherapy also showed meaningful improvements in progression-free survival (PFS) and overall response rate (ORR).
Keytruda, combined with Roche’s Herceptin (trastuzumab) and chemotherapy, was approved by the FDA last year under the accelerated pathway for first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric or GEJ adenocarcinoma. This accelerated approval was based on data from the phase III KEYNOTE-811 study, wherein treatment with this combination achieved a statistically significant ORR of 74%.
Merck’s shares have risen 39.5% this year compared with the industry’s 6.7% growth.
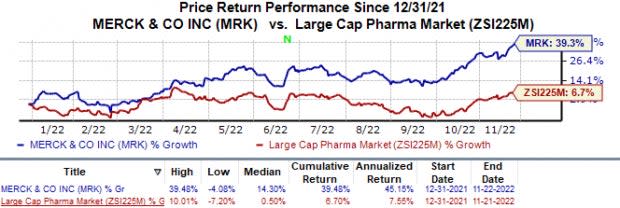
Image Source: Zacks Investment Research
Per management, advanced gastric cancer has one of the lowest five-year survival rates. The results from the KEYNOTE-859 study show that Keytruda plus chemotherapy improves survival beyond chemotherapy in HER2-negative patients. Merck is also evaluating Keytruda in an extensive clinical development program in gastrointestinal cancers, including a first-line advanced HER-2 positive gastric cancer and early-stage gastric cancer study
Other than gastric cancer, Keytruda is approved for the treatment of many cancers globally. Keytruda sales are gaining from continued strong momentum in metastatic indications, including in some types of NSCLC, renal cell carcinoma, head and neck squamous cell carcinoma, TNBC and MSI-H cancers. Keytruda is continuously growing and expanding into new indications and markets globally. In the first nine months of 2022, Merck generated $15.5 billion from Keytruda sales.
Keytruda faces stiff competition from Bristol Myers’ BMY Opdivo, which is also approved for many cancer indications. Combined with chemotherapy, Bristol Myers’ Opdivo is also approved by the FDA under the accelerated pathway for treating patients with advanced or metastatic gastric cancer, GEJ cancer and esophageal adenocarcinoma.
Opdivo is one of the many blockbuster drugs marketed by Bristol Myers. In the first nine months of 2022, Bristol Myers generated $6.0 billion from Opdivo sales. The drug is one of the key top-line drivers for Bristol Myers.
Merck & Co., Inc. Price
Merck & Co., Inc. price | Merck & Co., Inc. Quote
Zacks Rank & Stocks to Consider
Merck currently carries a Zacks Rank #2 (Buy). Some better-ranked stocks in the overall healthcare sector include Angion Biomedica ANGN and Vertex Pharmaceuticals VRTX, each of which carries a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Angion Biomedica’s 2022 loss per share have narrowed from $1.64 to $1.54. During the same period, the loss estimates per share for 2023 have narrowed from $1.54 to $1.48. Shares of Angion Biomedica have plunged 70.7% in the year-to-date period.
Earnings of Angion Biomedica beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 66.42%, on average. In the last reported quarter, ANGN delivered an earnings surprise of 41.67%.
Vertex’s stock has risen 46.4% this year so far. While Vertex’s earnings estimates for 2022 have risen from $14.21 to $14.61 per share in the past 30 days, estimates for 2023 have increased from $15.12 to $15.60 per share during the same period.
Vertex beat earnings estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 3.16%. In the last reported quarter, Vertex reported an earnings surprise of 8.67%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Angion Biomedica Corp. (ANGN) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 