Medtronic (MDT) Progresses in SCS With FDA Nod for Vanta
Medtronic plc MDT continues to grab headlines with recent breakthroughs within its neuromodulation line. Following last week’s FDA approval of SenSight Directional Lead System, this time the company has announced another regulatory go-ahead related to its Vanta implantable neurostimulator (INS).
Vanta is a spinal cord stimulation implant for chronic pain. This high-performance recharge-free INS has a long life of up to 11 years. At comparable settings, this device claims to offer nearly twice the device life than competitive primary cell devices. To note, the Vanta neurostimulator represents a 10% increase in longevity compared to Medtronic's previous generation recharge-free device, PrimeAdvanced.
Vanta utilizes Medtronic's proprietary AdaptiveStim technology for personalized pain relief. AdaptiveStim technology automatically adjusts stimulation to maintain each patient's optimal dose.
Vanta also provides full-body MRI access using Medtronic SureScan technology. This increases the suitability of Vanta, considering the fact that nearly 82% of spinal chord stimulation (SCS) implanted patients will need at least one MRI within five years of implant.
Even with increased longevity, Vanta is 20% smaller than Medtronic’s PrimeAdvanced neurostimulator. The Vanta system also provides access to Snapshot reporting, Medtronic's proprietary data insights solution.
Industry Prospects
Per a Emergen Research Report published in Intrado GlobeNewswire, the global Neurostimulation devices market is expected to reach $13.70 billion by 2027, witnessing a CAGR of 12.6%. Factors like the frequent occurrence of motor and neurological disorders along with the growing acceptance of minimally invasive surgeries are expected to drive the market.
Given the market potential, the approval has been timed well.
Medtronic's Recent Strides in Neuromodulation Space
As noted earlier, last week, Medtronic received a major breakthrough following the FDA approval and consequent first U.S. implants of its SenSight Directional Lead System. SenSight is a unique kind of Deep Brain Stimulation (DBS) directional lead that for the first time combines the benefits of directionality with the power of sensing. SenSight claims to deliver precise, patient-specific DBS therapy for the treatment of some symptoms associated with diseases like Parkinson’s, dystonia and essential tremor as well as medically refractory epilepsy.
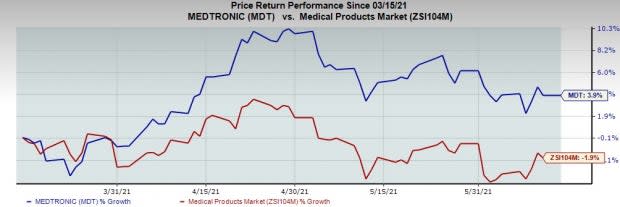
Image Source: Zacks Investment Research
In Neuroscience portfolio, in March 2021, Medtronic received CE Mark for SenSight directional lead system for DBS therapy as a treatment for symptoms associated with movement disorders and epilepsy.
In January, the company announced the first enrollment in ADAPT-PD (Adaptive DBS Algorithm for Personalized Therapy in Parkinson's Disease), its trial evaluating the safety and efficacy of adaptive deep brain stimulation (aDBS) in patients with Parkinson's Disease (PD).
Currently, Medtronic awaits the launch of its Vanta recharge-free spinal cord stimulator in the first half of fiscal 2022. It is also on track to submit the ECAPS device to the FDA later in the calendar year 2021.
Share Price Performance
The stock has outperformed its industry over the past three months. It has risen 3.9% against the industry’s 1.9% drop.
Zacks Rank and Key Picks
Currently, the company carries a Zacks Rank #3 (Hold).
A few better-ranked stocks from the broader medical space are Envista Holdings Corporation NVST, Inogen, Inc INGN and IDEXX Laboratories, Inc. IDXX, each carrying a Zacks Rank #2 (Buy). You can see the complete list of Zacks #1 Rank (Strong Buy) stocks here.
Envista Holdings has an estimated long-term earnings growth rate of 26%.
Inogen has an estimated long-term earnings growth rate of 33%.
IDEXX Laboratories has a projected long-term earnings growth rate of 20%.
Bitcoin, Like the Internet Itself, Could Change Everything
Blockchain and cryptocurrency has sparked one of the most exciting discussion topics of a generation. Some call it the “Internet of Money” and predict it could change the way money works forever. If true, it could do to banks what Netflix did to Blockbuster and Amazon did to Sears. Experts agree we’re still in the early stages of this technology, and as it grows, it will create several investing opportunities.
Zacks’ has just revealed 3 companies that can help investors capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 3 crypto-related stocks now >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Medtronic PLC (MDT) : Free Stock Analysis Report
IDEXX Laboratories, Inc. (IDXX) : Free Stock Analysis Report
Inogen, Inc (INGN) : Free Stock Analysis Report
Envista Holdings Corporation (NVST) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 