Mallinckrodt Completes NDA Submission for Kidney Failure Drug
Mallinckrodt plc MNK announced that it has completed the rolling submission of the new drug application (“NDA”) seeking approval for its pipeline candidate, terlipressin, as a treatment for hepatorenal syndrome type 1 (HRS-1). The life-threatening syndrome involves acute kidney failure in cirrhosis patients. The company initiated the rolling submission last month. The company stated that the NDA submission is considered as a Class 2 resubmission.
Per the FDA guidelines, the review of a Class 2 resubmission is completed within six months of acceptance. The FDA’s acceptance of the terlipressin NDA is yet to be announced.
The NDA included data from the phase III CONFIRM study, which evaluated terlipressin in patients suffering the HRS-1 syndrome. The study was conducted by the company under FDA’s special protocol assessment. Data from the study presented at The Liver Meeting 2019 showed that treatment with terlipressin achieved the study’s primary endpoint of Verified HRS Reversal in statistically significant number of patients compared to placebo. The Verified HRS Reversal is defined as renal function improvement, avoidance of dialysis and short-term survival.
Terlipressin is not approved for any indication in the United States and Canada. However, it is approved for treating HRS-1 outside these two countries.
A potential approval to terlipressin in the United States will be encouraging for the company as the HRS-1 syndrome is estimated to affect 30,000-40,000 people every year. Moreover, there are no FDA-approved therapy and more than 80% of patients die, if not treated for three months. These factors suggest a significant unmet need in the targeted indication.
Shares of Mallinckrodt have lost 51.6% so far this year compared with the industry’s decline of 22.7%.

Apart from terlipressin, the company has another key pipeline candidate, StrataGraft. The company has successfully completed a phase III study, evaluating the regenerative skin tissue candidate for treatment of severe, deep partial thickness burns in 2019. The company plans to file a biologics license application seeking approval for StrataGraft in the first half of 2020. Another mid-stage study is evaluating the candidate in patients with severe, full thickness burns.
Meanwhile, Mallinckrodt received a major boost with the announcement that it has reached an agreement in principle on the terms of a global settlement that would resolve all opioid-related claims against the company, Specialty Generics, and other subsidiaries. The company’s proposed global opioid settlement is currently supported by 48 states and territories.
Moreover, the company has also joined the race for a treatment for coronavirus alone with other pharma/biotech companies including J&J JNJ, Gilead GILD and Moderna MRNA. It is evaluating potential role of inhaled nitric oxide as a supportive measure in treating COVID-19 infection. It markets INOmax (nitric oxide) gas for inhalation in the United States for the treatment of hypoxic respiratory failure associated with pulmonary hypertension in newborns.
Mallinckrodt public limited company Price
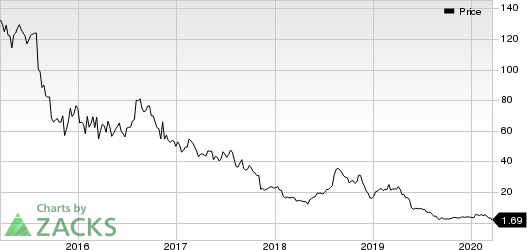
Mallinckrodt public limited company price | Mallinckrodt public limited company Quote
Zacks Rank
Mallinckrodt is a Zacks Rank #3 (Hold) stock currently. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Mallinckrodt public limited company (MNK) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 