Deciphera (DCPH) Rises 90% in the Past 6 Months: Here's Why
Shares of Deciphera Pharmaceuticals, Inc. DCPH have rallied 90% in the past six months against the industry’s decrease of 14.4%.
The company’s sole marketed drug, Qinlock (ripretinib), is approved for the treatment of adult patients with advanced gastrointestinal stromal tumor (“GIST”), who have received prior treatment with three or more kinase inhibitors, including Novartis’ Gleevec (imatinib).
Qinlock has also been approved in Europe for the treatment of fourth-line GIST, which is already generating incremental sales for the company.
DCPH has made steady progress with Qinlock during this time frame, with the drug witnessing solid uptake so far. Qinlock contributes the majority of Deciphera’s revenues.
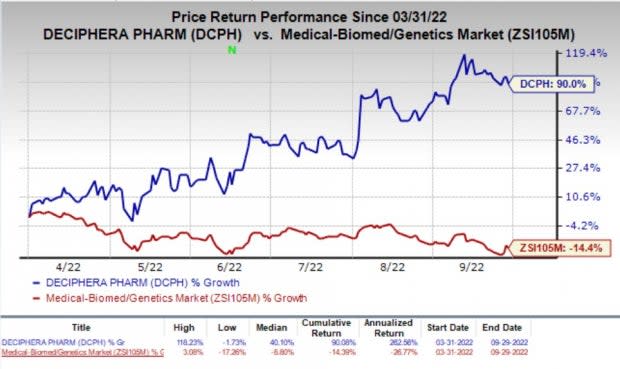
Image Source: Zacks Investment Research
The initial uptake of Qinlock has been strong since its approval in May 2020. In the first six months of 2022, Qinlock generated sales worth $60.3 million, reflecting an increase of 43.5% year over year.
DCPH is also working to expand the label of Qinlock for the larger commercial opportunity in GIST. A potential label expansion for other indications will make the drug eligible to treat a broader patient population and drive sales further in the days ahead.
This apart, Deciphera has a diverse pipeline and is advancing multiple drug candidates in various stages of clinical development.
The company is also evaluating vimseltinib in the phase III MOTION study for the treatment of patients with tenosynovial giant-cell tumors, who are not amenable to surgery.
A phase I study is evaluating DCC-3116 as a single agent and the company is also planning to initiate three phase early-stage studies on DCC-3116 in combination with the FDA-approved MEK inhibitor, trametinib, binimetinib as well as sotorasib, an FDA approved KRASG12C inhibitor, for treating patients with advanced or metastatic solid tumors later in 2022.
The successful development and potential approval of these candidates will be a big boost for the company and is likely to reduce its heavy dependence on Qinlock for revenues.
Deciphera currently has no approved product in its portfolio other than Qinlock. All of its candidates, including vimseltinib and DCC-3116, are still some time away from commercialization. As a result, the company is heavily dependent on Qinlock for growth. Any regulatory setback for the drug will hurt the company’s prospects.
Qinlock also faces competition from Blueprint Medicines’ BPMC Ayvakit, which is approved for the treatment of unresectable or metastatic GIST, harboring a PDGFRA exon 18 mutation, including PDGFRA D842V mutations in adults.
Blueprint Medicines’ lead-marketed drug Ayvakit holds great potential. BPMC is also studying the drug in various label expansion studies for additional indications.
Deciphera Pharmaceuticals, Inc. Price

Deciphera Pharmaceuticals, Inc. price | Deciphera Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Deciphera currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include Aptose Biosciences Inc. APTO and ORIC Pharmaceuticals, Inc. ORIC, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Aptose Biosciences’ loss per share estimates narrowed 15.7% for 2022 and 15% for 2023 in the past 60 days.
Earnings of Aptose Biosciences surpassed estimates in three of the trailing four quarters and missed on the remaining occasion. APTO delivered an earnings surprise of 2.23%, on average.
ORIC Pharmaceuticals’ loss per share estimates narrowed 8.6% for 2022 and 22% for 2023 in the past 60 days.
Earnings of ORIC Pharmaceuticals surpassed estimates in three of the trailing four quarters and missed on the remaining occasion. ORIC delivered an earnings surprise of 8.85%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Blueprint Medicines Corporation (BPMC) : Free Stock Analysis Report
Aptose Biosciences, Inc. (APTO) : Free Stock Analysis Report
Deciphera Pharmaceuticals, Inc. (DCPH) : Free Stock Analysis Report
Oric Pharmaceuticals, Inc. (ORIC) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 