Biogen's (BIIB) MS Drug, Vumerity, Gets Positive CHMP Opinion
Biogen Inc. BIIB announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (“CHMP”) has rendered a positive opinion on, and has recommended marketing authorization to Vumerity (diroximel fumarate) for the treatment of adults with relapsing-remitting multiple sclerosis (“RRMS”). The opinion will now be reviewed by the European Commission.
The positive CHMP opinion was based on data from pharmacokinetic bridging studies that compared Vumerity to Biogen’s another multiple sclerosis (“MS”) drug, Tecfidera.
The CHMP assessed data from the multi-center, double-blind phase III EVOLVE-MS-2 study, which evaluated the gastrointestinal (“GI”) tolerability profile of Vumerity versus Tecfidera in patients with RRMS. Data from the same showed that the rate of overall treatment discontinuation was lower in patients who were treated with Vumerity compared with those who received Tecfidera. The treatment discontinuation rate due to GI tolerability was 0.8% in the Vumerity arm compared to 4.8% in the Tecfidera arm.
Vumerity, an oral fumarate, was approved in the United States for the treatment of relapsing forms of MS in adults in 2019. In the first six months of 2021, Vumerity recorded sales worth $164.6 million.
In late 2017, Biogen in-licensed worldwide commercialization rights to Vumerity from Alkermes ALKS.
Shares of Biogen have rallied 22.6% so far this year compared with the industry’s growth of 0.9%.
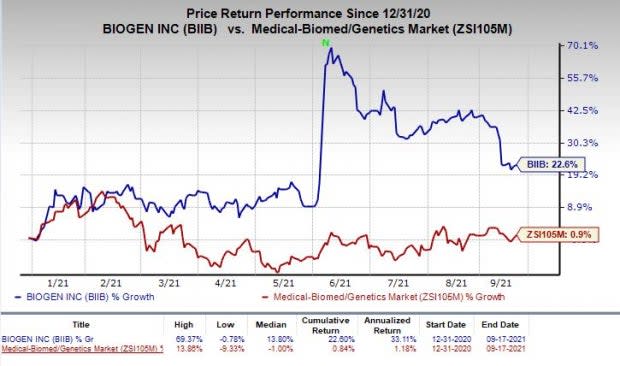
Image Source: Zacks Investment Research
Biogen holds a strong position in the MS market, with a wide range of products, including Avonex, Tysabri, Tecfidera and Plegridy. However, multiple generic versions of Tecfidera have been launched, which are significantly eroding the drug’s sales.
The market remains challenging for Biogen’s MS products with newer, competitive entrants. The launch of Ocrevus, a new MS drug by Roche RHHBY, is adversely impacting sales of Biogen’s MS franchise. However, Biogen receives royalties on U.S. sales of Ocrevus.
Biogen is working on consolidating its position in the MS market by bringing in new treatments.
Zacks Rank & Key Pick
Biogen currently carries a Zacks Rank #3 (Hold). A top-ranked stock in the biotech sector is Regeneron Pharmaceuticals, Inc. REGN, which has a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Regeneron’s earnings estimates have been revised 17.8% upward for 2021 and 13.8% upward for 2022 over the past 60 days. The stock has surged 35% year to date.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Alkermes plc (ALKS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 