Alnylam's (ALNY) Marketed Drugs Aid Growth Amid Competition
Alnylam Pharmaceuticals, Inc. ALNY is making good progress with its portfolio of marketed drugs – Onpattro (patisiran), Givlaari (givosiran) and Oxlumo (lumasiran).
The company generates revenues from product sales, licensing and strategic collaborations, as well as royalties.
Alnylam's lead drug, Onpattro, is approved for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. The company is also planning to expand the label of Onpattro in ATTR amyloidosis patients with cardiomyopathy.
Alnylam’s second product, Givlaari, was approved for the treatment of acute hepatic porphyria in the United States in November 2019 and in Europe in March 2020.
Oxlumo injection for subcutaneous use was approved in November 2020 for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary oxalate levels in pediatric and adult patients. Oxlumo is Alnylam’s third product.
In March 2022, the FDA accepted the supplemental new drug application for Oxlumo for the reduction of plasma oxalate in the treatment of patients with advanced primary hyperoxaluria type 1. A decision from the regulatory body is expected on Oct 6, 2022. A potential label expansion of the drug is likely to boost sales further.
Shares of Alnylam have lost 24.3% so far this year compared with the industry’s decline of 22.9%.
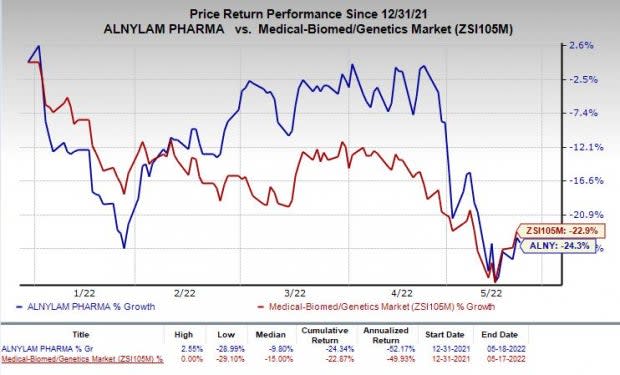
Image Source: Zacks Investment Research
In December 2021, Alnylam’s partner, Novartis NVS, announced that the FDA had approved Leqvio (inclisiran) to reduce low-density lipoprotein cholesterol (LDL-C) with two doses a year. Leqvio has also been approved by the European Commission to treat hypercholesterolemia.
Alnylam is entitled to receive tiered royalties on the global sales of Leqvio from NVS.
Novartis has obtained global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam.
This apart, the FDA has accepted the new drug application for Alnylam’s investigational RNAi therapeutic, vutrisiran, being developed for the treatment of adult patients with polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. A decision from the regulatory body is expected on Jul 14, 2022.
Regulatory applications seeking approval of vutrisiran for a similar indication are also under review in Europe, Japan and Brazil.
Alnylam has a robust pipeline of candidates that it is evaluating for various indications.
Though Alnylam is making steady progress with its portfolio of marketed drugs, stiff competition in the target market remains a constant threat. Several other companies are also developing RNAi-based therapeutics. This can induce acute competition for Alnylam in the days ahead.
Alnylam Pharmaceuticals, Inc. Price and Consensus
Alnylam Pharmaceuticals, Inc. price-consensus-chart | Alnylam Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Alnylam currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are Applied Therapeutics, Inc. APLT and Aeglea BioTherapeutics, Inc. AGLE, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate of Applied Therapeutics’ loss per share has narrowed 27.1% for 2022 and 20.2% for 2023 over the past 60 days.
Earnings of Applied Therapeutics have surpassed estimates in one of the trailing four quarters, met the same once and missed the same on the other two occasions, delivering an earnings surprise of -0.67%, on average.
Aeglea BioTherapeutics’ loss per share estimates have narrowed 23.2% for 2022 and 30.6% for 2023 over the past 60 days.
Earnings of Aeglea BioTherapeutics have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions, delivering an earnings surprise of 9.47%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Aeglea BioTherapeutics, Inc. (AGLE) : Free Stock Analysis Report
Applied Therapeutics Inc. (APLT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 