Aerie (AERI) Earnings Miss in Q2, Rhopressa Performs Well
Aerie Pharmaceuticals, Inc. AERI reported second-quarter 2018 loss of $1.14 per share, wider than both the Zacks Consensus Estimate of a loss of $1.02 and the year-ago loss of 63 cents. The wider-than-expected loss was on account of higher operating costs related to the launch of the lead drug.
Aerie Pharmaceuticals, Inc. Price, Consensus and EPS Surprise

Aerie Pharmaceuticals, Inc. Price, Consensus and EPS Surprise | Aerie Pharmaceuticals, Inc. Quote
Quarter in Detail
In December 2017, Aerie's lead drug, Rhopressa was approved by the FDA for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. This approval came two months ahead of the scheduled Prescription Drug User Fee Act date of Feb 28, 2018. The drug was launched by the end of April.
Rhopressa’s revenues came in at $2.4 million, beating the Zacks Consensus Estimate of $1.16 million.
In the reported quarter, research and development expenses increased 69.5% to $15.6 million. Selling, general and administrative expenses surged to $32.1 million from $11.9 million in the year-ago quarter.
Also, operating expenses were higher in the reported quarter primarily due to increased activities associated with the expansion of the employee base to support the growth of operations and activities associated with Rhopressa commercialization efforts.
Aerie is securing formulary contracts to enable commercial coverage in 2018 and Medicare Part D coverage in 2019. As of Aug 1, 2018, Rhopressa’s market access increased to approximately 80% (up from 70% as last reported) of commercial lives including 55% in Tier 3 and 25% in preferred brand Tier 2. Medicare Part D coverage is now 12% (up from 10% as last reported) in Tier 2.
Pipeline Updates
Aerie’s New Drug Application (NDA) for its second product candidate, Roclatan (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005%, which is a fixed-dose combination of Rhopressa and Pfizer’s PFE Xalatan, was submitted to the FDA in May 2018. The company received the “Day 74” notification from the FDA, earlier than scheduled date. The agency has completed its initial 60-day review of the NDA and determined that the application is sufficiently complete to permit a substantive review. The PDUFA (Prescription Drug User Fee Act) goal date for the completion of the FDA’s review of the Roclatan NDA is set for Mar 14, 2019. The “Day 74” notification indicates that the FDA has not identified any potential review issues and might not need an advisory committee review.
Meanwhile, the company initiated a phase III trial, Mercury 3, in the third quarter of 2017 to prepare for a regulatory submission in Europe. The trial is a non-inferiority trial comparing Roclatan with prescribed fixed-dose combination of Ganfort.
Pre-IND activities are well underway for further development of Aerie’s retina program candidates including AR-13503 (Rho kinase and Protein kinase C inhibitor implant) and AR-1105 (dexamethasone steroid implant).
Outlook Reiterated
Aerie expects Rhopressa’s revenues in the range of $20-$30 million in 2018. Total cash burn is projected in the range of $200-$210 million.
Our Take
The wider Q2 loss was primarily due to higher expenses as Aerie strives to commercialize Rhopressa. Nevertheless, Rhopressa sales comfortably beat estimates. The solid uptake in prescription volumes should propel sales further as glaucoma is one of the largest segments in the global ophthalmic market, even though competition is stiff from the likes of Bausch Health's BHC Vyzulta among others.
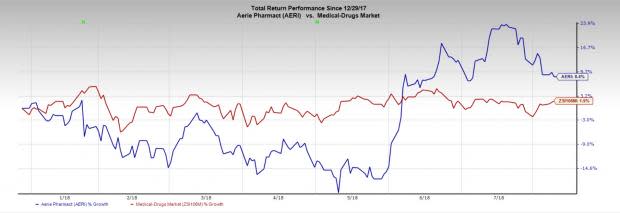
Aerie’s shares have returned 8.4% in the year so far compared with the industry’s gain of 1.9%.
Finally, a tentative approval of Roclatan (early next year) will further boost the prospects of the company.
Zacks Rank & Key Pick
Aerie carries a Zacks Rank #2 (Buy).
Another attractive stock in the healthcare sector is Gilead Sciences, Inc. GILD, which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Gilead’s earnings per share estimates increased from $6.10 to $6.57 for 2018 over the last 60 days. Estimates for 2019 are also up by 14 cents.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Aerie Pharmaceuticals, Inc. (AERI) : Free Stock Analysis Report
Bausch Health Cos Inc. (BHC) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance