AbbVie's RA Candidate Meets Primary Endpoints in Phase III
AbbVie Inc. ABBV has announced that its pipeline candidate, upadacitinib, met the primary endpoints in the 4th phase III study from its SELECT program, which evaluated the candidate for the treatment of patients with rheumatoid arthritis (RA). Upadacitinib is an investigational oral JAK1-selective inhibitor, which is being studied as a once-a-day oral therapy.
The phase III SELECT-COMPARE study is designed to evaluate the efficacy and safety of upadacitinib (15 mg) compared with placebo and AbbVie’s marketed drug, Humira (adalimumab), over 12 weeks on patients with moderate-to-severe RA, who are on a stable background of methotrexate and had an inadequate response. In the study, upadacitinib met both the primary as well as secondary endpoints compared with placebo while showing superiority as compared to Humira.
The primary endpoints of the study registered ACR20 responses and clinical remission. Data from the trial demonstrated that 71% of patients receiving the 15 m oral once-daily dose of upadacitinib 15 mg achieved an ACR20 response compared with 36% of patients receiving placebo at week 12. Moreover, 29% of subjects treated with upadacitinib achieved complete remission in comparison to 6% with placebo.
Additionally, data from the study also showed that 45% of patients experienced low disease activity (secondary endpoint) when treated with upadacitinib compared with 29% receiving Humira and 14%, receiving placebo at week 12.
Notably, SELECT-COMPARE is the fourth of the six ongoing phase III studies under the SELECT RA clinical trial program conducted on upadacitinib.
We remind investors that last December, AbbVie announced that upadacitinib met the primary endpoints in the 3rd phase III study from its SELECT trial.
Other researches are also underway on upadacitinib for treating Crohn’s disease, ulcerative colitis and atopic dermatitis.
Shares of AbbVie have rallied 42.7% in the past year, outperforming the industry’s 6.4% rise.

Notably, AbbVie already has a strong presence in the RA market with its blockbuster drug, Humira. However, several companies are working on developing biosimilar versions of Humira which could induce competition in the market for the company. Also, the RA market is extremely crowded including drugs like Johnson & Johnson’s Simponi and UCB’s Cimzia among others.
Significantly, Eli Lilly’s LLY JAK inhibitor, Olumiant, was approved in the EU for RA last February and Pfizer’s PFE Xeljanz is marketed in the United States for the same indication, which might pose a strong competition for upadacitinib on approval.
Also, of late, Biogen BIIB has announced resolution of the ongoing patent dispute with AbbVie regarding Biogen’s biosimilar version of Humira, in the EU. Per the settlement agreement, Biogen plans to launch Humira biosimilar in Europe by October, 2018.
However, if upadacitinib is approved, it might reduce the potential negative impact of Humira generics on AbbVie’s top line.
AbbVie Inc. Price
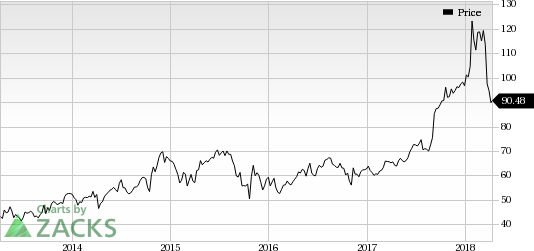
AbbVie Inc. Price | AbbVie Inc. Quote
Zacks Rank
AbbVie carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks Rank #1 (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance